Arzneimittel-Forschung
2000-07-01
Effect of topically applied dexpanthenol on epidermal barrier function and stratum corneum hydration. Results of a human in vivo study.
W Gehring, M Gloor
Index: Arzneimittelforschung 50(7) , 659-63, (2000)
Full Text: HTML
Abstract
In a randomized, double-blind, placebo-controlled study the effect of topical dexpanthenol (CAS 81-13-0) formulated in two different lipophilic vehicles on epidermal barrier function in vivo was carried out. Seven days' treatment with dexpanthenol improved stratum corneum hydration and reduced transepidermal water loss. Active treatment was statistically different from the vehicle control on both measures. Our results suggest that topical dexpanthenol formulated in either lipophilic vehicle stabilizes the skin barrier function.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
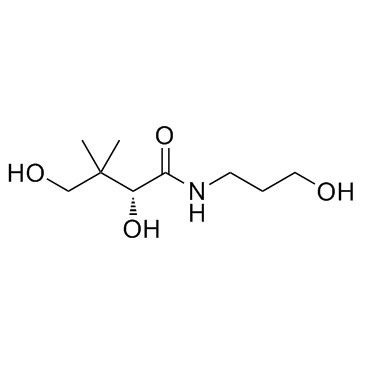 |
Dexpanthenol
CAS:81-13-0 |
C9H19NO4 |
Related Articles:
More...
|
Release of betaine and dexpanthenol from vitamin E modified ...
2015-03-01 [Curr. Eye Res. 40(3) , 267-73, (2015)] |
|
Cystoid macular oedema following Descemet membrane endotheli...
2015-01-01 [Br. J. Ophthalmol. 99(1) , 98-102, (2015)] |
|
Characterization of a novel standardized human three-dimensi...
2015-03-01 [Lasers Surg. Med. 47(3) , 257-65, (2015)] |
|
Topical use of dexpanthenol in skin disorders.
2002-01-01 [Am. J. Clin. Dermatol. 3(6) , 427-33, (2002)] |
|
[The metabolism of panthenol in patients with postoperative ...
1990-12-01 [Z. Ernahrungswiss. 29(4) , 270-83, (1990)] |