| Structure | Name/CAS No. | Articles |
|---|---|---|
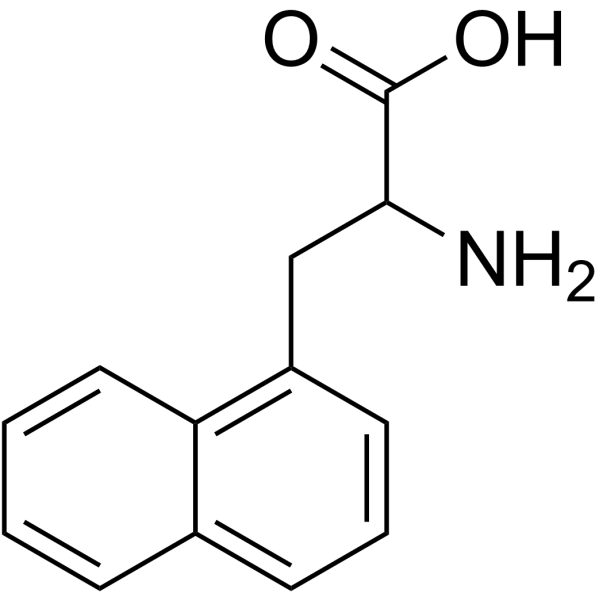 |
1-Naphthalenealanine
CAS:28095-56-9 |
S Hanessian, N Bernstein, R Y Yang, R Maguire
Index: Bioorg. Med. Chem. Lett. 9(10) , 1437-42, (1999)
Full Text: HTML
Enantiopure L-azetidine-2-carboxylic acid, the (3R)-phenyl, (3R)-naphthyl and (3S)-isopropyl analogs were prepared based on a zinc-mediated asymmetric addition of allylic halides to the camphor sultam derivative of glyoxylic acid O-benzyl oxime.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
1-Naphthalenealanine
CAS:28095-56-9 |
C13H13NO2 |
|
Synthesis and characterization of more potent analogues of h...
1998-09-29 [Biochemistry 37(39) , 13507-15, (1998)] |
|
The A14 position of insulin tolerates considerable structura...
1992-10-01 [J. Protein Chem. 11(5) , 571-7, (1992)] |
|
Adding L-3-(2-Naphthyl)alanine to the genetic code of E. col...
2002-03-06 [J. Am. Chem. Soc. 124 , 1836-1837, (2002)] |
|
Conformational analyses of cyclic hexapeptide analogs of som...
1999-04-01 [J. Pept. Sci. 5(4) , 161-75, (1999)] |
|
Synthesis and binding properties of cyclohexapeptide somatos...
1999-03-01 [J. Pept. Sci. 5(3) , 113-30, (1999)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved