Synthesis and binding properties of cyclohexapeptide somatostatin analogs containing naphthylalanine and arylalkyl peptoid residues.
T A Tran, R H Mattern, B A Morgan, J E Taylor, M Goodman
Index: J. Pept. Sci. 5(3) , 113-30, (1999)
Full Text: HTML
Abstract
We report the synthesis, binding affinities to the recombinant human somatostatin receptors, and structure-activity relationship studies of compounds related to the cyclic hexapeptide, c-[Pro6-Phe7-D-Trp8Lys9-Thr10-Phe11], L-363,301 (the numbering in the sequence refers to the position of the residues in native somatostatin). The Pro residue in this compound is replaced with the arylalkyl peptoid residues Nphe (N-benzylglycine), (S)betaMeNphe [(S)-N-[alpha(-methyl)benzyl]glycine] or (R)betaMeNphe [(R)-N-[(alpha-methyl)benzyl]glycine] and L-1-naphthylalanine is incorporated into either position 7 or 11 of the parent compound. The synthesis and binding data of the Nnal6 ([N-naphthylmethyl]glycine) analog of L-363,301 is also reported. The incorporation of the Nnal residue into position 6 of L-363,301 resulted in an analog with weaker binding affinities to all hsst receptors but enhanced selectivity towards the hsst2 receptor compared with the parent compound. The other compounds bind effectively to the hsst2 receptor but show some variations in the binding to the hsst3 and hsst5 receptors resulting in different ratios of binding affinities to the hsst5 and hsst2 or hsst3 and hsst2, respectively. The incorporation of the Nphe residue into position 6 and the Nal residue into position 7 of L-363,301 led to a compound which binds potently to the hsst2 and has increased selectivity towards this receptor (weaker binding to hsst3 and hsst5 receptors) compared with the parent compound. The analogs with beta-methyl chiral substitutions in the aromatic peptoid side chain and Nal in position 7 or 11 bind effectively to the hsst2 and hsst5 receptors. They exhibit similar ratios of binding affinities to the hsst5 and hsst2 receptors as observed for L-363,301. There are however minor differences in binding to the hsst3 receptor among these analogs. These studies allow us to investigate the influence of additional hydrophobic groups on the binding activity to the isolated human somatostatin receptors and the results are important for the design of other somatostatin analogs.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
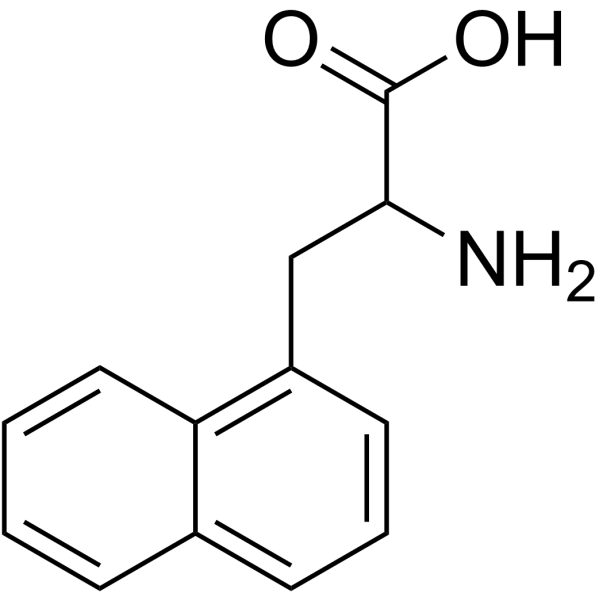 |
1-Naphthalenealanine
CAS:28095-56-9 |
C13H13NO2 |
|
Synthesis and characterization of more potent analogues of h...
1998-09-29 [Biochemistry 37(39) , 13507-15, (1998)] |
|
The A14 position of insulin tolerates considerable structura...
1992-10-01 [J. Protein Chem. 11(5) , 571-7, (1992)] |
|
Adding L-3-(2-Naphthyl)alanine to the genetic code of E. col...
2002-03-06 [J. Am. Chem. Soc. 124 , 1836-1837, (2002)] |
|
Conformational analyses of cyclic hexapeptide analogs of som...
1999-04-01 [J. Pept. Sci. 5(4) , 161-75, (1999)] |
|
The conformation of linear gramicidin is sequence dependent....
1994-01-01 [Eur. Biophys. J. 22(6) , 447-52, (1994)] |