Plasma estriol and its conjugates following oral and vaginal administration of estriol to postmenopausal women: correlations with gonadotropin levels.
I Schiff, D Tulchinsky, K J Ryan, S Kadner, M Levitz
Index: Am. J. Obstet. Gynecol. 138(8) , 1137-41, (1980)
Full Text: HTML
Abstract
A study was designed to compared the metabolic fate and the biologic effects of 4 mg of estriol (E3) administered either orally or vaginally to six postmenopausal women. Blood samples were collected every hour for 6 hours and five different estriol fractions as well as gonadotropins were measured. Vaginal E3 administration resulted in a decline of 45% in luteinizing hormone (LH) levels and 17% in follicle-stimulating hormone (FSH) levels at 6 hours after treatment (p < 0.05). In contrast, the administration of 4 mg of E3 orally did not produce a decline of LH and FSH, despite the fact that the serum levels of E3-3-sulfate, E3-3-sulfate-16-glucosiduronate, estriol-3-glucosiduronate, and estriol-16-glucosiduronate were all fourfold to 24-fold higher after oral administration than after vaginal estriol administration. However, since the levels of unconjugated E3 were higher after the vaginal than after the oral administration of estriol, we conclude that only unconjugated E3 suppresses gonadotropins.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
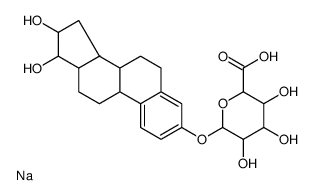 |
Estriol 3-β-D-Glucuronide (sodium salt)
CAS:15087-06-6 |
C24H32NaO9 |
|
Klotho is a novel beta-glucuronidase capable of hydrolyzing ...
2004-03-12 [J. Biol. Chem. 279(11) , 9777-84, (2004)] |
|
Urinary estrone conjugate and pregnanediol 3-glucuronide enz...
2003-07-01 [Clin. Chem. 49(7) , 1139-48, (2003)] |
|
Estriol and its conjugates in late pregnancy determined by e...
1985-10-01 [Clin. Chem. 31(10) , 1698-702, (1985)] |
|
Direct determination of estriol 3- and 16-glucuronides in pr...
2003-06-01 [Biomed. Chromatogr. 17(4) , 219-25, (2003)] |
|
Direct determination of estriol conjugates in amniotic fluid...
2006-01-01 [Rapid Commun. Mass Spectrom. 20(19) , 2995-8, (2006)] |