The metabolism of estriol-3-glucosiduronate and estriol in the rabbit.
T Miyazaki, H Mizukoshi, Y Araki, N Shimizu
Index: Endocrinol. Jpn. 27(2) , 175-82, (1980)
Full Text: HTML
Abstract
Urinary metabolites of [6,7-3H]-estriol-3-glucosiduronate and of [6, 7-3H]-estriol in intact female rabbits were analyzed. The separation of urinary metabolites was performed by countercurrent distribution followed by DEAE-Sephadex A-25 column chromatography. Each conjugate was then hydrolyzed with the enzymes and the aglycone thus liberated was identified. In either case, major urinary metabolites were found to be diconjugates, a considerable part of which was glucosiduronate-N-acetylglucosaminide of 17-epiestriol. In addition, estriol-16-glucosiduronate or monoglucosiduronate of 17-epiestriol was identified as a minor urinary metabolite of [6,7-3H]-estriol. From these results, it was concluded that the greater part of the estriol-3-glucosiduronate was converted to diconjugates and that estriol-3-glucosiduronate was probably an intermediate metabolite in the conversion pathway from estriol to diconjugates in this species.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
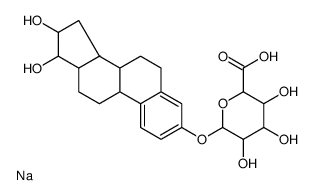 |
Estriol 3-β-D-Glucuronide (sodium salt)
CAS:15087-06-6 |
C24H32NaO9 |
|
Klotho is a novel beta-glucuronidase capable of hydrolyzing ...
2004-03-12 [J. Biol. Chem. 279(11) , 9777-84, (2004)] |
|
Urinary estrone conjugate and pregnanediol 3-glucuronide enz...
2003-07-01 [Clin. Chem. 49(7) , 1139-48, (2003)] |
|
Estriol and its conjugates in late pregnancy determined by e...
1985-10-01 [Clin. Chem. 31(10) , 1698-702, (1985)] |
|
Direct determination of estriol 3- and 16-glucuronides in pr...
2003-06-01 [Biomed. Chromatogr. 17(4) , 219-25, (2003)] |
|
Direct determination of estriol conjugates in amniotic fluid...
2006-01-01 [Rapid Commun. Mass Spectrom. 20(19) , 2995-8, (2006)] |