| Structure | Name/CAS No. | Articles |
|---|---|---|
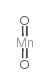 |
Stannic oxide
CAS:18282-10-5 |
|
 |
Cobalt
CAS:7440-48-4 |
|
 |
Ferric oxide
CAS:1309-37-1 |
|
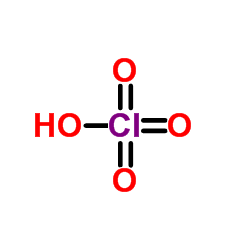 |
PERCHLORIC ACID
CAS:7601-90-3 |
|
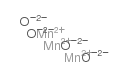 |
Manganese(II,III) oxide
CAS:1317-35-7 |