Water-soluble L-alanine and related oligopeptide conjugates with poly[(R,S)-3-hydroxybutanoic acid] oligomers, synthesis and structural studies by means of electrospray ionization multistage mass spectrometry.
Z G Arkin, J Rydz, G Adamus, M Kowalczuk
Index: J. Biomater. Sci. Polym. Ed. 12(3) , 297-305, (2001)
Full Text: HTML
Abstract
The reactions of (R,S) beta-butyrolactone with L-alanine and related oligopeptides (Ala-Ala-Ala) were investigated. The resulting water-soluble oligomers were composed of poly[(R,S)-3-hydroxybutanoic acid] (a-PHB) covalently conjugated to L-alanine and Ala-Ala-Ala oligopeptide. The other chain end was of the carboxylic acid type. The structure of the obtained oligomers was assessed by electrospray ionization multistage mass spectrometry (ESI-MSn) and the respective structural information was completed by IR, NMR, and GPC analyses. The molecular weight and structure of the products could be controlled through reaction conditions. Using this new synthetic approach. a-PHB oligomers with well-defined end groups, as well as respective block copolymers, can be prepared via regioselective ring-opening oligomerization of (R,S) beta-butyrolactone induced by amino acids under their zwitterionic form.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
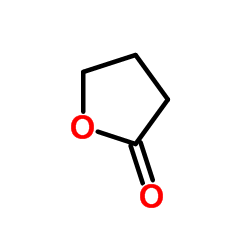 |
butyrolactone
CAS:3068-88-0 |
C4H6O2 |
|
Involvement of catalytic amino acid residues in enzyme-catal...
2001-07-01 [Biomacromolecules 2(2) , 541-4, (2001)] |
|
Ring-opening polymerisation of beta-butyrolactone catalysed ...
1999-01-01 [Int. J. Biol. Macromol. 25(1-3) , 237-45, (1999)] |
|
Novel synthesis of functionalized poly(3-hydroxybutanoic aci...
1999-01-01 [Int. J. Biol. Macromol. 25(1-3) , 247-53, (1999)] |
|
Demixing of severely overlapped ¹H NMR resonances and interp...
[J. Magn. Reson. 207(2) , 190-6, (2010)] |
|
Highly controlled immortal polymerization of β-butyrolactone...
2012-07-11 [Chem. Commun. (Camb.) 48(54) , 6806-8, (2012)] |