Ring-opening polymerisation of beta-butyrolactone catalysed by distannoxane complexes: study of the mechanism.
Y Hori, T Hagiwara
Index: Int. J. Biol. Macromol. 25(1-3) , 237-45, (1999)
Full Text: HTML
Abstract
The mechanism of the ring-opening polymerisation of beta-butyrolactones was studied. The ring-opening polymerisation of BL catatlysed by distannoxane complexes is of a living nature. The polymerisation of racemic BL gave a predominantly syndiotactic P(3HB). The temperature effect on syndiospecificity was used to determine the activation energy (deltaE = Esyndiotactic - Eisotactic) for syndiotactic versus isotactic diad placement. The deltaE value was obtained as -1.49 kcal/mol. The steric control leading to the observed syndiospecificity is due predominantly to diastereomeric interactions between the Sn-coordinated P(3HB) chain end. having a specific chain end stereochemistry, and the incoming BL enantiomeric monomers. The catalytic cycle derived from the mechanism of the polymerisation was proposed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
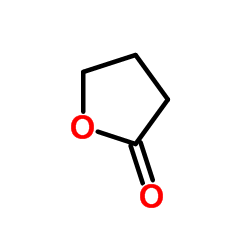 |
butyrolactone
CAS:3068-88-0 |
C4H6O2 |
|
Involvement of catalytic amino acid residues in enzyme-catal...
2001-07-01 [Biomacromolecules 2(2) , 541-4, (2001)] |
|
Novel synthesis of functionalized poly(3-hydroxybutanoic aci...
1999-01-01 [Int. J. Biol. Macromol. 25(1-3) , 247-53, (1999)] |
|
Demixing of severely overlapped ¹H NMR resonances and interp...
[J. Magn. Reson. 207(2) , 190-6, (2010)] |
|
Water-soluble L-alanine and related oligopeptide conjugates ...
2001-01-01 [J. Biomater. Sci. Polym. Ed. 12(3) , 297-305, (2001)] |
|
Highly controlled immortal polymerization of β-butyrolactone...
2012-07-11 [Chem. Commun. (Camb.) 48(54) , 6806-8, (2012)] |