| Structure | Name/CAS No. | Articles |
|---|---|---|
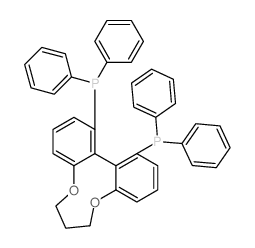 |
(R)-C3-TUNAPHOS
CAS:301847-89-2 |
|
![[Chloro(R)-C3-TunePhos)(p-cymene)ruthenium(II)] chloride Structure](https://image.chemsrc.com/caspic/391/905709-79-7.png) |
[Chloro(R)-C3-TunePhos)(p-cymene)ruthenium(II)] chloride
CAS:905709-79-7 |
|
![(S)-1,13-Bis(diphenylphosphino)-7,8-dihydro-6H-dibenzo[f,h][1,5]dioxonine Structure](https://image.chemsrc.com/caspic/325/486429-99-6.png) |
(S)-1,13-Bis(diphenylphosphino)-7,8-dihydro-6H-dibenzo[f,h][1,5]dioxonine
CAS:486429-99-6 |