| Structure | Name/CAS No. | Articles |
|---|---|---|
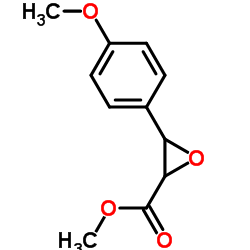 |
Methyl 3-(4-methoxyphenyl)oxirane-2-carboxylate
CAS:42245-42-1 |
|
 |
(E)-3-(4-Methoxyphenyl)acrylic acid
CAS:943-89-5 |
|
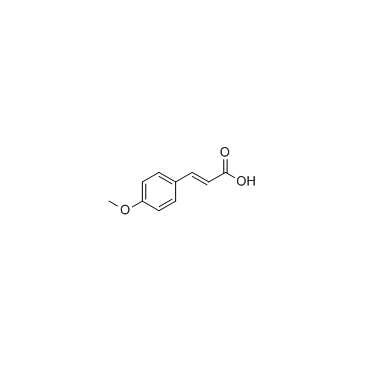 |
4-Methoxycinnamic acid
CAS:830-09-1 |