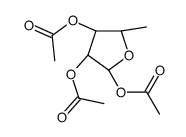
|
~87% |
|
~72% |
|
~71% |
|
~% |
|
~44% |
|
~94% |
|
~78% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |



![((3aR,4R,6R,6aR)-6-Methoxy-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methanol Structure](https://image.chemsrc.com/caspic/093/4099-85-8.png)

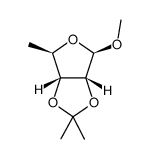
![2-methoxy-7,7-dimethyl-4-(methylsulfonyloxymethyl)-3,6,8-trioxabicyclo[3.3.0]octane Structure](https://image.chemsrc.com/caspic/016/50610-99-6.png)