Structure/function correlations among coupled binuclear copper proteins through spectroscopic and reactivity studies of NspF.
Jake W Ginsbach, Matthew T Kieber-Emmons, Ryohei Nomoto, Akio Noguchi, Yasuo Ohnishi, Edward I Solomon
Index: Proc. Natl. Acad. Sci. U. S. A. 109(27) , 10793-7, (2012)
Full Text: HTML
Abstract
The terminal step of 4-hydroxy-3-nitrosobenzamide biosynthesis in Streptomyces murayamaensis is performed by NspF, a mono-oxygenase that converts o-aminophenols to the corresponding nitroso product (hydroxyanilinase activity). Previous biochemical characterization of the resting form of NspF suggested that this enzyme belonged to the coupled binuclear copper enzyme (CBC) family. Another member of this enzyme family, tyrosinase, is able to mono-oxygenate monophenols (monophenolase activity) but not o-aminophenols. To gain insight into the unique reactivity of NspF, we have generated and characterized the oxy form of its active site. The observation of spectral features identical to those of oxy-tyrosinase indicates that oxy-NspF contains a Cu(2)O(2) core where peroxide is coordinated in a μ-η(2):η(2) mode, confirming that NspF is a CBC enzyme. This oxy form is found to react with monophenols, indicating that, like tyrosinase, NspF also possesses monophenolase activity. A comparison of the two electrophilic mechanisms for the monophenolase and hydroxyanilinase activity indicates a large geometric change between their respective transition states. The potential for specific interactions between the protein pocket and the substrate in each transition state is discussed within the context of the differential reactivity of this family of enzymes with equivalent μ-η(2):η(2) peroxy bridged coupled binuclear copper active sites.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
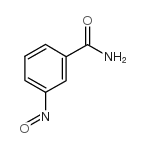 |
3-nitrosobenzamide
CAS:144189-66-2 |
C7H6N2O2 |
|
The site of antiviral action of 3-nitrosobenzamide on the in...
1993-10-15 [Proc. Natl. Acad. Sci. U. S. A. 90 , 9721, (1993)] |
|
Inhibition of the replication of native and 3'-azido-2',3'-d...
1993-07-12 [FEBS Lett. 326 , 140, (1993)] |
|
A copper-containing oxidase catalyzes C-nitrosation in nitro...
2010-09-01 [Nat. Chem. Biol. 6(9) , 641-3, (2010)] |
|
Anti-HIV agents that selectively target retroviral nucleocap...
1998-04-23 [J. Med. Chem. 41(9) , 1371-81, (1998)] |
|
Inhibition of HIV-1 infectivity by zinc-ejecting aromatic C-...
1993-02-04 [Nature 361 , 473, (1993)] |