| Structure | Name/CAS No. | Articles |
|---|---|---|
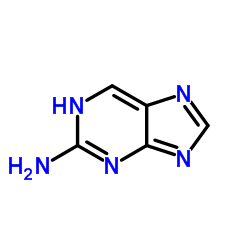 |
2-Aminopurine
CAS:452-06-2 |
|
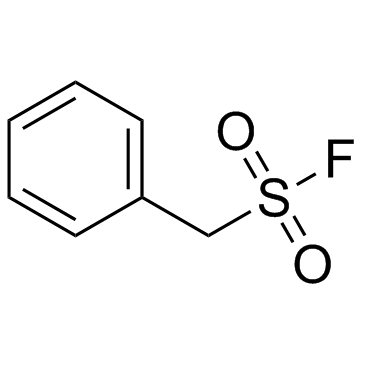 |
PMSF
CAS:329-98-6 |
|
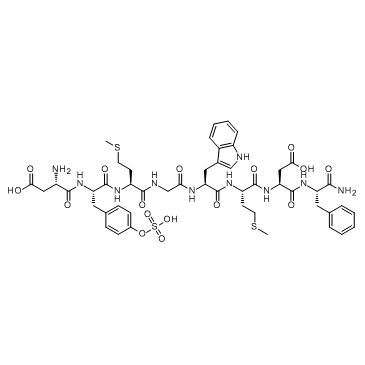 |
Cholecystokinin Octapeptide (sulfated) ammonium salt
CAS:25126-32-3 |
|
 |
Polyinosinic-polycytidylic acid sodium
CAS:42424-50-0 |