| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
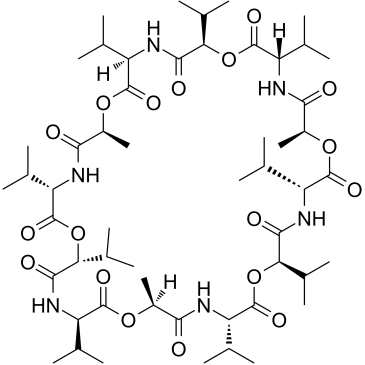 |
Valinomycin
CAS:2001-95-8 |
|
 |
Adamantan-1-amine
CAS:768-94-5 |
|
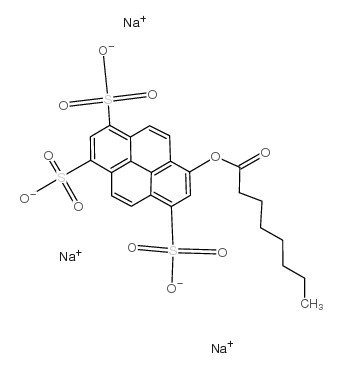 |
8-Octanoyloxypyrene-1,3,6-trisulfonic acid trisodium salt
CAS:115787-84-3 |
|
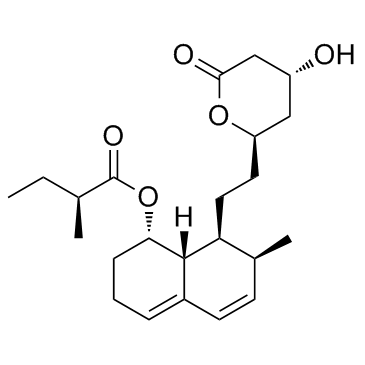 |
Mevastatin
CAS:73573-88-3 |