| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
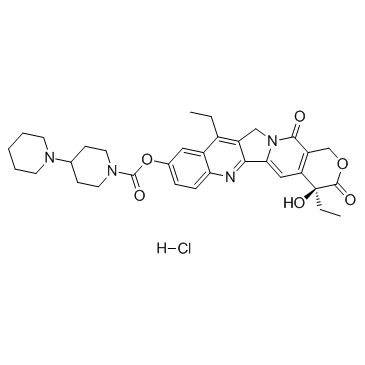 |
Irinotecan hydrochloride
CAS:100286-90-6 |
|
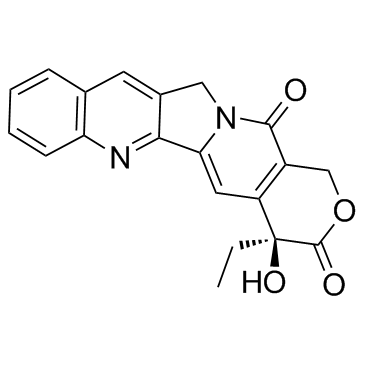 |
Campathecin
CAS:7689-03-4 |
|
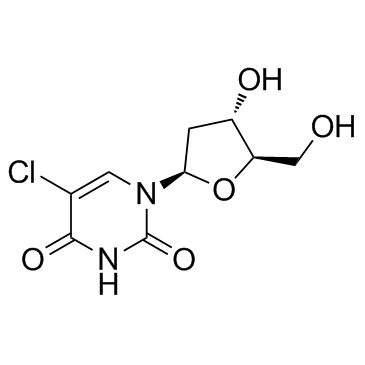 |
5-Chloro-2'-deoxyuridine
CAS:50-90-8 |
|
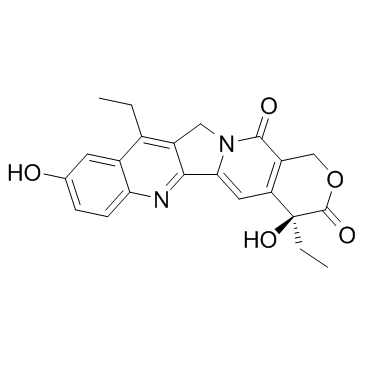 |
7-Ethyl-10-hydroxycamptothecin
CAS:86639-52-3 |
|
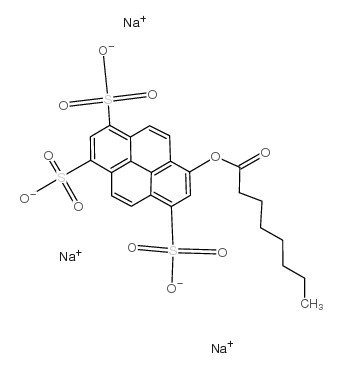 |
8-Octanoyloxypyrene-1,3,6-trisulfonic acid trisodium salt
CAS:115787-84-3 |