| Structure | Name/CAS No. | Articles |
|---|---|---|
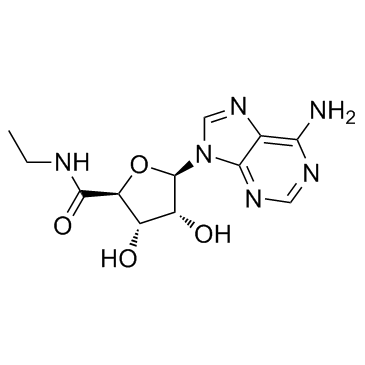 |
NECA
CAS:35920-39-9 |
|
 |
ccpa
CAS:37739-05-2 |
|
 |
DPCPX
CAS:102146-07-6 |