InI₃- or ZnI₂-catalyzed reaction of hydroxylated 1,5-allenynes with thiols: a new access to 3,5-disubstituted toluene derivatives.
Jie Ma, Lingling Peng, Xiu Zhang, Zhe Zhang, Melody Campbell, Jianbo Wang
Index: Chem. Asian J. 5(10) , 2214-20, (2010)
Full Text: HTML
Abstract
Transition-metal-activated alkynes or allenes can accept nucleophilic attack and undergo direct addition of the nucleophiles to the unsaturated bonds or trigger subsequent rearrangement reactions. This chemistry has witnessed increasing development in recent years. In this report, we have focused on the metal-catalyzed reactions of a variety of substituted propargyl allenic alcohols and thiophenols using indium(III) and zinc(II) catalysts, which can activate both the alcohol and alkyne. In this reaction, thio groups play the role of a nucleophile and trigger subsequent rearrangements to give benzene derivatives. The products can be further transformed into various 1,3,5-trisubstituted aromatic compounds by nickel-catalyzed coupling reactions through the cleavage of the C-S bonds.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
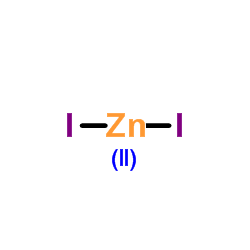 |
Zinc iodide
CAS:10139-47-6 |
I2Zn |
|
Synthesis of fluorenone and anthraquinone derivatives from a...
2013-09-20 [Org. Lett. 15(18) , 4888-91, (2013)] |
|
Ambipolar zinc-polyiodide electrolyte for a high-energy dens...
2015-01-01 [Nat. Commun. 6 , 6303, (2015)] |
|
Novel (cyanamide)Zn(II) complexes and zinc(ii)-mediated hydr...
2014-11-14 [Dalton Trans. 43(42) , 15798-811, (2014)] |
|
ZIO impregnation and cytochemical localization of thiamine p...
1994-10-01 [Histol. Histopathol. 9(4) , 649-56, (1994)] |
|
An efficient synthesis of 2,3-dideoxy-alpha,beta-unsaturated...
2006-06-12 [Carbohydr. Res. 341(8) , 1052-6, (2006)] |