Carbohydrate Research
2006-06-12
An efficient synthesis of 2,3-dideoxy-alpha,beta-unsaturated carbohydrate enals by mixed Lewis acid (HfCl4 and ZnI2) catalyzed hydration of glycals.
Mohammad Saquib, Ram Sagar, Arun K Shaw
Index: Carbohydr. Res. 341(8) , 1052-6, (2006)
Full Text: HTML
Abstract
A new, efficient method has been developed for converting acyl-, arylalkyl- and alkyl-protected glycals into corresponding 2,3-dideoxy-alpha,beta-unsaturated carbohydrate enals utilizing the in situ generated push-pull effect resulting from the synergistic combination of HfCl4 and ZnI2 in catalytic amounts. This new procedure eliminates the use of highly toxic Hg2+ ions and acidic conditions (0.01-0.02 N H2SO4), besides radically shortening the reaction time.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
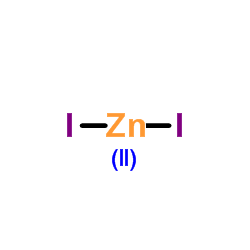 |
Zinc iodide
CAS:10139-47-6 |
I2Zn |
Related Articles:
More...
|
Synthesis of fluorenone and anthraquinone derivatives from a...
2013-09-20 [Org. Lett. 15(18) , 4888-91, (2013)] |
|
Ambipolar zinc-polyiodide electrolyte for a high-energy dens...
2015-01-01 [Nat. Commun. 6 , 6303, (2015)] |
|
Novel (cyanamide)Zn(II) complexes and zinc(ii)-mediated hydr...
2014-11-14 [Dalton Trans. 43(42) , 15798-811, (2014)] |
|
ZIO impregnation and cytochemical localization of thiamine p...
1994-10-01 [Histol. Histopathol. 9(4) , 649-56, (1994)] |
|
Ultrastructure of the zinc iodide-osmic acid stained cells i...
1995-10-01 [J. Anat. 187 ( Pt 2) , 481-5, (1995)] |