| Structure | Name/CAS No. | Articles |
|---|---|---|
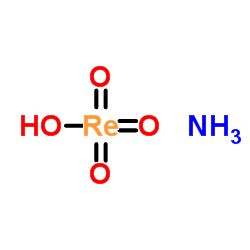 |
ammonium perrhenate
CAS:13598-65-7 |
|
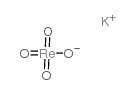 |
Potassium perrhenate
CAS:10466-65-6 |
|
 |
Sodium perrhenate, 99.99% metals basis
CAS:13472-33-8 |