| Structure | Name/CAS No. | Articles |
|---|---|---|
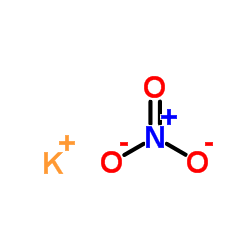 |
Potassium Nitrate
CAS:7757-79-1 |
|
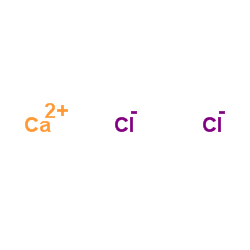 |
Calcium chloride
CAS:10043-52-4 |
|
 |
magnesium sulfate
CAS:7487-88-9 |
|
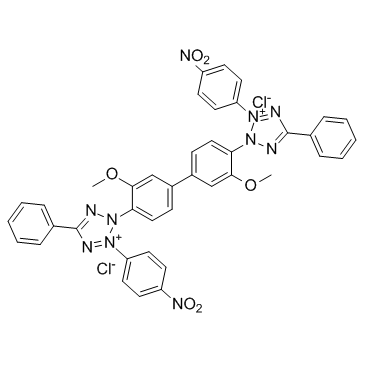 |
NBT
CAS:298-83-9 |
|
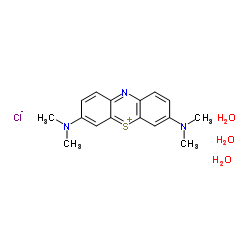 |
Methylene Blue trihydrate
CAS:7220-79-3 |
|
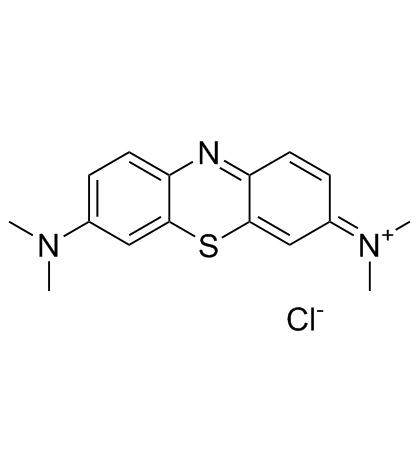 |
Methylene Blue
CAS:61-73-4 |
|
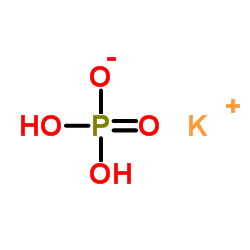 |
Monopotassium phosphate
CAS:7778-77-0 |
|
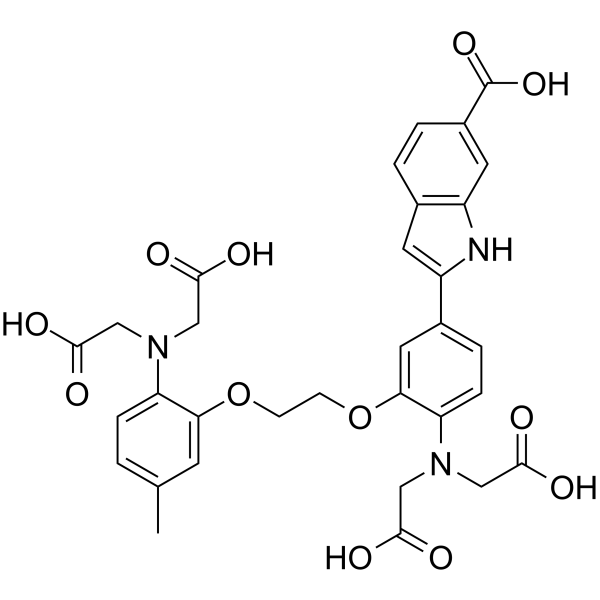 |
Indo 1
CAS:96314-96-4 |
|
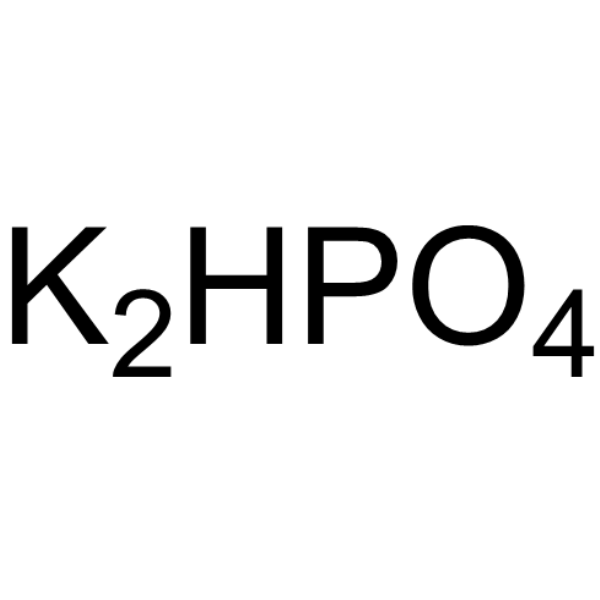 |
Di-potassium monohydrogen phosphate
CAS:7758-11-4 |
|
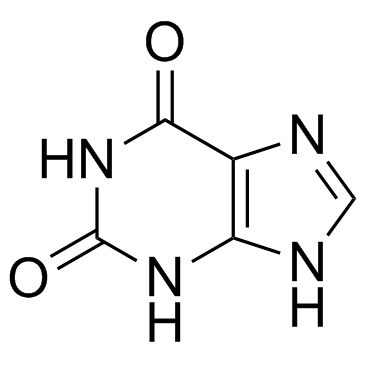 |
2,6-Dihydroxypurine
CAS:69-89-6 |