Prospective serial evaluation of 2-hydroxyglutarate, during treatment of newly diagnosed acute myeloid leukemia, to assess disease activity and therapeutic response.
Amir T Fathi, Hossein Sadrzadeh, Darrell R Borger, Karen K Ballen, Philip C Amrein, Eyal C Attar, Julia Foster, Meghan Burke, Hector U Lopez, Christina R Matulis, Katherine M Edmonds, A John Iafrate, Kimberly S Straley, Katharine E Yen, Samuel Agresta, David P Schenkein, Cedric Hill, Ashkan Emadi, Donna S Neuberg, Richard M Stone, Yi-Bin Chen
Index: Blood 120(23) , 4649-52, (2012)
Full Text: HTML
Abstract
Mutations of genes encoding isocitrate dehydrogenase (IDH1 and IDH2) have been recently described in acute myeloid leukemia (AML). Serum and myeloblast samples from patients with IDH-mutant AML contain high levels of the metabolite 2-hydroxyglutarate (2-HG), a product of the altered IDH protein. In this prospective study, we sought to determine whether 2-HG can potentially serve as a noninvasive biomarker of disease burden through serial measurements in patients receiving conventional therapy for newly diagnosed AML. Our data demonstrate that serum, urine, marrow aspirate, and myeloblast 2-HG levels are significantly higher in IDH-mutant patients, with a correlation between baseline serum and urine 2-HG levels. Serum and urine 2-HG, along with IDH1/2-mutant allele burden in marrow, decreased with response to treatment. 2-HG decrease was more rapid with induction chemotherapy compared with DNA-methyltransferase inhibitor therapy. Our data suggest that serum or urine 2-HG may serve as noninvasive biomarkers of disease activity for IDH-mutant AML.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
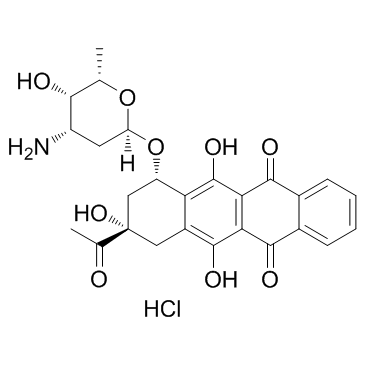 |
Idarubicin (hydrochloride)
CAS:57852-57-0 |
C26H28ClNO9 |
|
Breakthrough Fusarium solani infection in a patient with acu...
2014-06-01 [Ann. Hematol. 93(6) , 1079-81, (2014)] |
|
A yeast-based assay identifies drugs that interfere with imm...
2014-04-01 [Dis. Model Mech. 7(4) , 435-44, (2014)] |
|
Final report of phase II study of sorafenib, cytarabine and ...
2014-07-01 [Leukemia 28(7) , 1543-5, (2014)] |
|
HLA-partially matched cellular therapy (stem-cell microtrans...
2014-05-01 [Br. J. Haematol. 165(4) , 580-1, (2014)] |
|
A randomized trial of prophylactic palifermin on gastrointes...
2014-12-01 [Br. J. Haematol. 167(5) , 618-25, (2014)] |