| Structure | Name/CAS No. | Articles |
|---|---|---|
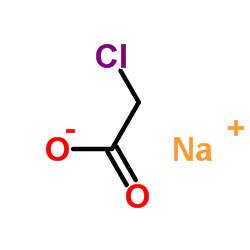 |
Sodium chloroacetate
CAS:3926-62-3 |
|
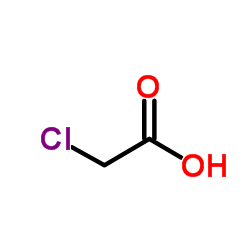 |
Chloroacetic acid
CAS:79-11-8 |
|
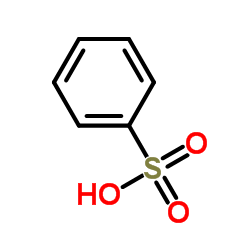 |
Benzenesulfonic acid
CAS:98-11-3 |
|
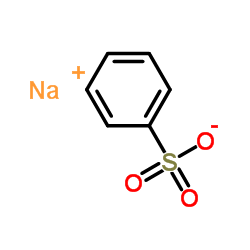 |
Sodium benzenesulfonate
CAS:515-42-4 |