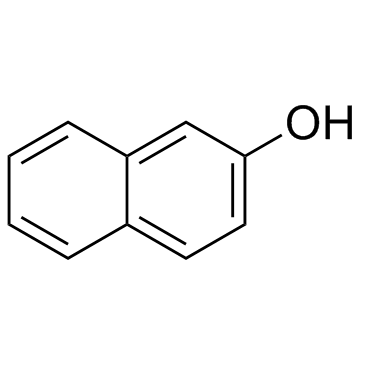
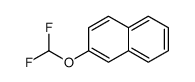
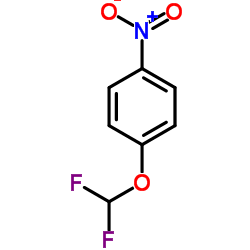
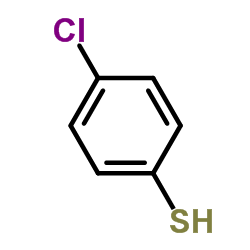
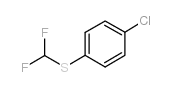
|
~88% |
|
~90% |
|
~92% |
|
~98% |
|
~76% |
|
~97% |
|
~88% |
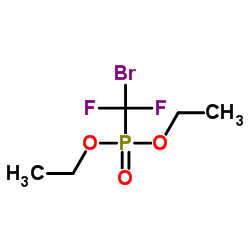

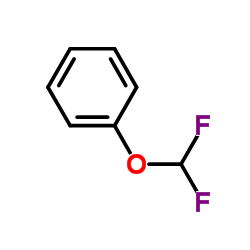

![[(Difluoromethyl)thio]benzene Structure](https://image.chemsrc.com/caspic/214/1535-67-7.png)
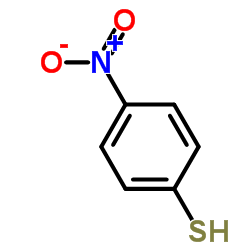
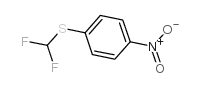
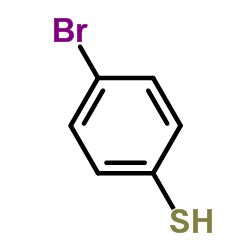
![1-Bromo-4-[(difluoromethyl)sulfanyl]benzene Structure](https://image.chemsrc.com/caspic/290/4837-14-3.png)