| Structure | Name/CAS No. | Articles |
|---|---|---|
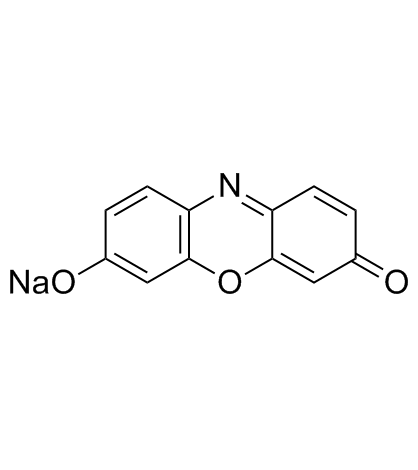 |
Resorufin sodium salt
CAS:34994-50-8 |
|
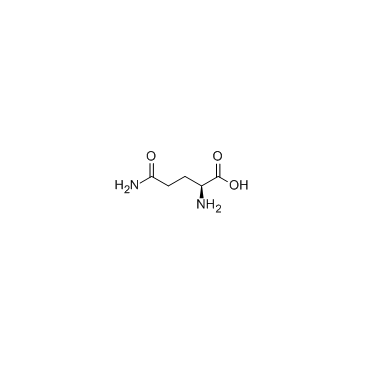 |
L-Glutamine
CAS:56-85-9 |
|
 |
HEPES
CAS:7365-45-9 |
|
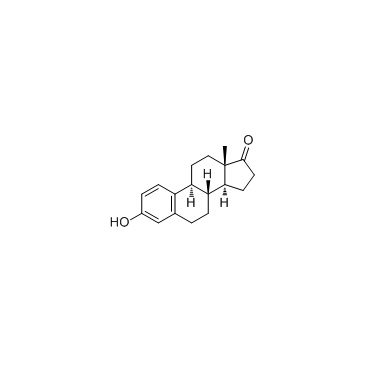 |
Estrone
CAS:53-16-7 |
|
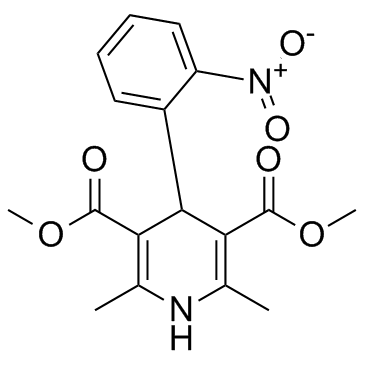 |
Nifedipine
CAS:21829-25-4 |
|
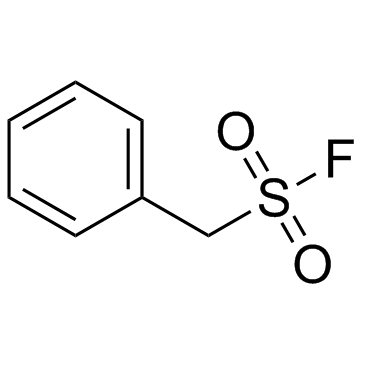 |
PMSF
CAS:329-98-6 |
|
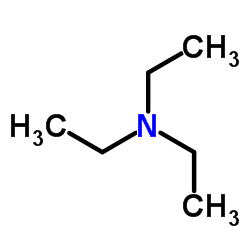 |
Triethylamine
CAS:121-44-8 |
|
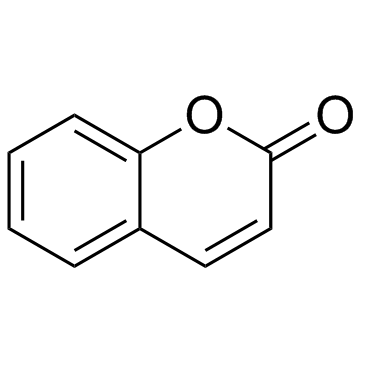 |
Coumarin
CAS:91-64-5 |
|
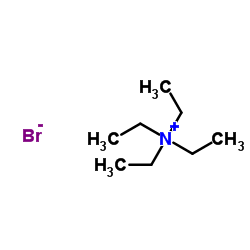 |
Tetraethylammonium bromide
CAS:71-91-0 |
|
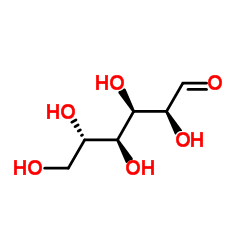 |
L-Glucose
CAS:921-60-8 |