| Structure | Name/CAS No. | Articles |
|---|---|---|
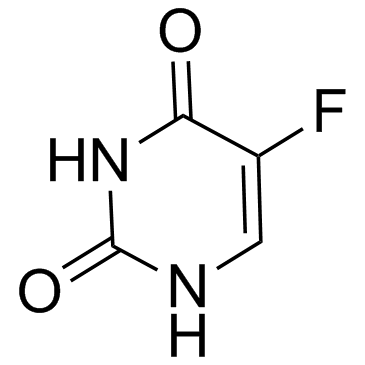 |
Fluorouracil
CAS:51-21-8 |
|
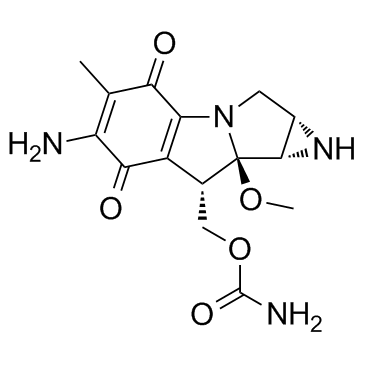 |
Mitomycin C
CAS:50-07-7 |
|
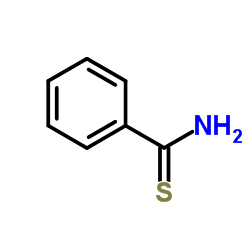 |
Thiobenzamide
CAS:2227-79-4 |