Comparison of sorption behavior of Th(IV) and U(VI) on modified impregnated resin containing quinizarin with that conventional prepared impregnated resin.
Mohammad Saeid Hosseini, Ahmad Hosseini-Bandegharaei
Index: J. Hazard. Mater. 190(1-3) , 755-65, (2011)
Full Text: HTML
Abstract
This paper reports the results obtained by studying the ion-exchange properties of a new solvent impregnated resin (SIR), which was prepared by impregnation of quinizarin (1,4-dihydroxyanthraquinone, QNZ) on Amberlite XAD-16 after nitration of the benzene rings present in its structure. The sorption behavior of Th(IV) and U(VI) on/in the modified SIR was compared with that of the SIR prepared via the conventional method. It was observed that sorption capacity and sorption rate of the modified SIR are significantly greater than the conventional one. The modified SIR was then applied to the extraction of Th(IV) and U(VI) ions at the presence of many co-existence metal ions. The results obtained denote on successful application of this new SIR to analysis of natural water samples spiked to Th(IV) and U(VI) ions.Copyright © 2011 Elsevier B.V. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
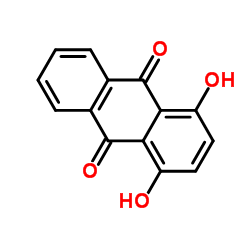 |
Quinizarin
CAS:81-64-1 |
C14H8O4 |
|
In vitro inhibition of Streptococcus mutans biofilm formatio...
2007-04-01 [Antimicrob. Agents Chemother. 51 , 1541-4, (2007)] |
|
Synthesis and activity of substituted anthraquinones against...
2005-04-21 [J. Med. Chem. 48 , 2822-30, (2005)] |
|
Synthesis of glycoside derivatives of hydroxyanthraquinone w...
2010-03-01 [Eur. J. Med. Chem. 45 , 1001-7, (2010)] |
|
Solvent effects on the photophysical properties of poly[1,4-...
2015-01-05 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 134 , 218-224, (2015)] |
|
An investigation of anthraquinone dye biodegradation by immo...
2012-06-01 [Environ. Sci. Pollut. Res. Int. 19(5) , 1728-37, (2012)] |