| Structure | Name/CAS No. | Articles |
|---|---|---|
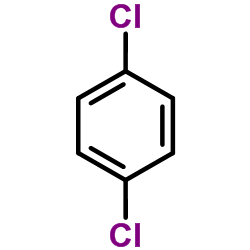 |
1,4-Dichlorobenzene
CAS:106-46-7 |
|
 |
Sulfur trioxide
CAS:7446-11-9 |