Conformational modification of muscle phosphofructokinase from Jaculus orientalis upon ligand binding.
M Tijane, Z el Hachimi, A Benjouad, M Desmadril, J M Yon
Index: FEBS Lett. 245(1-2) , 30-4, (1989)
Full Text: HTML
Abstract
Phosphofructokinase from Jaculus orientalis muscle is an allosteric enzyme regulated by substrates and nucleotide effectors. The conformational modifications upon ligand binding were probed by UV difference spectra and reactivities of thiol groups towards dithiobisnitrobenzoate and N-ethylmaleimide. The binding of Fru-6-P induced significant perturbations in the environment of the aromatic residues and buried the most reactive on the three accessible cysteines per protomer. The same effect on thiol reactivity was observed upon binding of the activator AMP. Various perturbations of both difference spectra and thiol reactivity were detected in the presence of either Mg-ATP, an allosteric inhibitor, or Mg-ITP which is not an effector.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
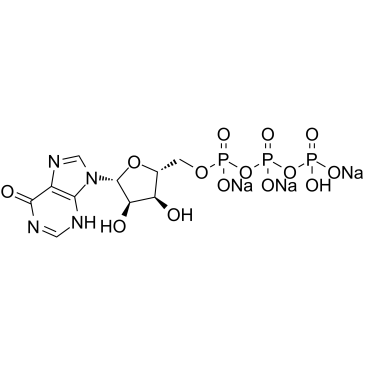 |
Inosine 5'-triphosphate (sodium salt)
CAS:35908-31-7 |
C10H12N4Na3O14P3 |
|
[Nucleoside-5'-triphosphate hydrolysis in the liver and kidn...
2006-01-01 [Biomed. Khim. 52 , 364-369, (2006)] |
|
Mapping interactions between the Ca2+-ATPase and its substra...
2003-03-21 [J. Biol. Chem. 278 , 10112-10118, (2003)] |
|
Identification of an ITPase/XTPase in Escherichia coli by st...
2005-10-01 [Structure 13 , 1511-1520, (2005)] |
|
Trinitrophenyl-ATP and -ADP bind to a single nucleotide site...
1988-04-25 [J. Biol. Chem. 263 , 5569-5573, (1988)] |
|
Distinct interactions of G(salpha-long), G(salpha-short), an...
2002-08-15 [Biochem. Pharmacol. 64 , 583-593, (2002)] |