Trinitrophenyl-ATP and -ADP bind to a single nucleotide site on isolated beta-subunit of Escherichia coli F1-ATPase. In vitro assembly of F1-subunits requires occupancy of the nucleotide-binding site on beta-subunit by nucleoside triphosphate.
R Rao, M K Al-Shawi, A E Senior
Index: J. Biol. Chem. 263 , 5569-5573, (1988)
Full Text: HTML
Abstract
The stoichiometry of nucleotide binding to the isolated alpha- and beta-subunits of Escherichia coli F1-ATPase was investigated using two experimental techniques: (a) titration with fluorescent trinitrophenyl (TNP) derivatives of AMP, ADP, and ATP and (b) the centrifuge column procedure using the particular conditions of Khananshvili and Gromet-Elhanan (Khananshvili, D., and Gromet-Elhanan, Z. (1985) FEBS Lett. 178, 10-14). Both procedures showed that alpha-subunit contains one nucleotide-binding site, confirming previous work. TNP-ADP and TNP-ATP bound to a maximal level of 1 mol/mol beta-subunit, consistent with previous equilibrium dialysis studies which showed isolated beta-subunit bound 1 mol of ADP or ATP per mol (Issartel, J. P., and Vignais, P. V. (1984) Biochemistry 23, 6591-6595). However, binding of only approximately 0.1 mol of ATP or ADP per mol of beta-subunit was detected using centrifuge columns. Our results are consistent with the conclusion that each of the alpha- and beta-subunits contains one nucleotide-binding domain. Because the subunit stoichiometry is alpha 3 beta 3 gamma delta epsilon, this can account for the location of the six known nucleotide-binding sites in E. coli F1-ATPase. Studies of in vitro assembly of isolated alpha-, beta-, and gamma- subunits into an active ATPase showed that ATP, GTP, and ITP all supported assembly, with half-maximal reconstitution of ATPase occurring at concentrations of 100-200 microM, whereas ADP, GDP, and IDP did not. Also TNP-ATP supported assembly and TNP-ADP did not. The results demonstrate that (a) the nucleotide-binding site on beta-subunit has to be filled for enzyme assembly to proceed, whereas occupancy of the alpha-subunit nucleotide-binding site is not required, and (b) that enzyme assembly requires nucleoside triphosphate.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
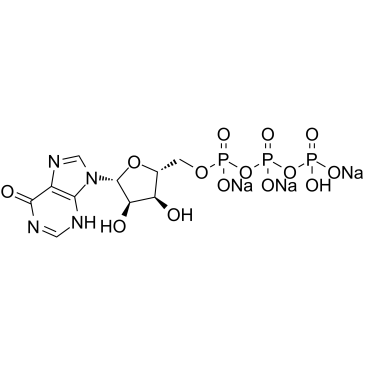 |
Inosine 5'-triphosphate (sodium salt)
CAS:35908-31-7 |
C10H12N4Na3O14P3 |
|
[Nucleoside-5'-triphosphate hydrolysis in the liver and kidn...
2006-01-01 [Biomed. Khim. 52 , 364-369, (2006)] |
|
Mapping interactions between the Ca2+-ATPase and its substra...
2003-03-21 [J. Biol. Chem. 278 , 10112-10118, (2003)] |
|
Identification of an ITPase/XTPase in Escherichia coli by st...
2005-10-01 [Structure 13 , 1511-1520, (2005)] |
|
Distinct interactions of G(salpha-long), G(salpha-short), an...
2002-08-15 [Biochem. Pharmacol. 64 , 583-593, (2002)] |
|
Characterization of an ecto-ATPase activity in Cryptococcus ...
2005-07-01 [FEMS Yeast Res. 5 , 899-907, (2005)] |