Reduced haloperidol does not interfere with the antipsychotic activity of haloperidol in the treatment of acute schizophrenia.
S Ulrich, S Neuhof, V Braun, F P Meyer
Index: Int. Clin. Psychopharmacol. 14(4) , 219-28, (1999)
Full Text: HTML
Abstract
The aim of this study was to investigate the effect of reduced haloperidol, the main metabolite of the antipsychotic drug haloperidol, on psychopathology improvement and extrapyramidal adverse effects in acute schizophrenia. The steady-state pharmacokinetics of reduced haloperidol was studied. Serum concentrations of reduced haloperidol (C(RH)) and haloperidol (C(H)) were measured in an open clinical trial over 6 weeks of treatment in 57 acutely schizophrenic patients. Psychopathology was measured by the Brief Psychiatric Rating Scale and several subscales. The assay of extrapyramidal adverse effects was conducted by means of the Extrapyramidal Symptom Rating Scale. A significant serum concentration-therapeutic effect relationship (SCTER) of haloperidol of the same data has been demonstrated. In our study, the influence of the metabolite reduced haloperidol on the antipsychotic activity of haloperidol was analysed by means of regression analysis of the residuals of the SCTER of haloperidol with C(RH). In addition, the steady-state pharmacokinetics of reduced haloperidol and direct relationships between C(RH) and the metabolite ratio C(RH)/C(H) with psychopathology improvement and extrapyramidal adverse effects were investigated. Reduced haloperidol was not found to interfere with the antipsychotic action of the parent drug. Patients with elevated C(RH) or elevated metabolite ratio C(RH)/C(H) did not show consistently lower clinical improvements compared with the fitting curve of the SCTER of haloperidol and therefore no significant relationship between C(RH) and the residuals of the SCTER of haloperidol was detected. Furthermore, no significant result was found in the analysis of the direct relationships of C(RH) and C(RH)/C(H) with clinical variables which, for example, indicate decreased outcome with increased C(RH). In contrast, because of the pharmacokinetic dependence of C(RH) and C(H), a trend for a bisigmoidal relationship with C(RH) emerged for some outcome variables which was traced as an epiphenomenon from the bisigmoidal SCTER of the parent drug (e.g. change of hostility after 3 weeks). No relationship of reduced haloperidol with extrapyramidal adverse effects could be detected. It is concluded that serum concentrations of reduced haloperidol are of minor value for the interpretation of data of therapeutic drug monitoring of haloperidol in patients with acute schizophrenia. Reduced haloperidol does not act as a 'false neuroleptic'.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
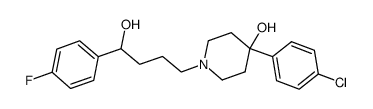 |
Haloperidol Metabolite II
CAS:34104-67-1 |
C21H25ClFNO2 |
|
Antagonism by haloperidol and its metabolites of mechanical ...
2009-07-01 [Psychopharmacology 205 , 21-33, (2009)] |
|
Determination of haloperidol and reduced haloperidol in huma...
2006-10-01 [Anal. Bioanal. Chem 386(3) , 719-24, (2006)] |
|
Plasma reduced haloperidol/haloperidol ratios in schizophren...
1994-06-01 [Eur. Neuropsychopharmacol. 4(2) , 119-26, (1994)] |
|
Correlation of clinical response (PANSS) and plasma levels o...
1994-03-01 [Prog. Neuropsychopharmacol. Biol. Psychiatry 18(2) , 347-53, (1994)] |
|
Effect of a genetic polymorphism of CYP1A2 inducibility on t...
2000-06-01 [Ther. Drug Monit. 22(3) , 245-9, (2000)] |