Efficient total synthesis of pulchellalactam, a CD45 protein tyrosine phosphatase inhibitor.
Wen-Ren Li, Sung Tsai Lin, Nai-Mu Hsu, Meei-Shiou Chern
Index: J. Org. Chem. 67(14) , 4702-6, (2002)
Full Text: HTML
Abstract
A new approach to a CD45 protein tyrosine phosphatase inhibitor, pulchellalactam, is described. The key step of the sequence involves addition and elimination of an enolic lactam in a single step and 70% yield, employing an organocuprate reagent. The resulting alpha,beta-unsaturated lactam could be condensed with isobutyraldehyde to produce Z-pulchellalactam or converted into siloxypyrrole, which was subjected to the BF(3) x Et(2)O-promoted coupling reaction with isobutyraldehyde to afford E-pulchellalactam after E1-cB elimination and TFA deprotection. This first total synthesis afforded Z-pulchellalactam in six steps and 32% overall yield from Boc-glycine. The same sequence of reactions could also be applied to the liquid- or solid-phase synthesis of trifunctionalized pulchellalactam derivatives.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
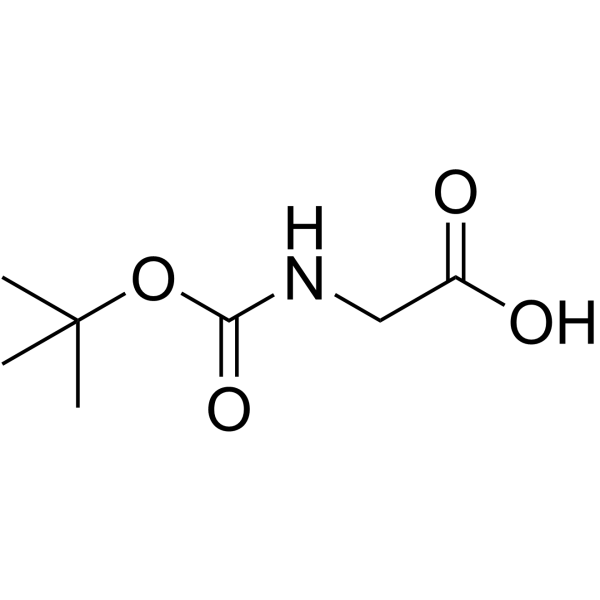 |
Boc-Gly-OH
CAS:4530-20-5 |
C7H13NO4 |
|
In situ fabrication of cleavable peptide arrays on polydimet...
2015-03-20 [Anal. Chim. Acta 865 , 53-9, (2015)] |
|
Accumulation of cell-penetrating peptides in large unilamell...
2015-09-01 [Biopolymers 104 , 533-43, (2015)] |
|
Optimization of the Ugi reaction using parallel synthesis an...
2008-01-01 [J. Vis. Exp. (21) , doi:10.3791/942, (2008)] |
|
Measurements of carbon to amide-proton distances by C-H dipo...
2003-05-14 [J. Am. Chem. Soc. 125(19) , 5648-9, (2003)] |
|
15N spin diffusion rate in solid-state NMR of totally enrich...
2006-10-01 [J. Magn. Reson. 182(2) , 339-42, (2006)] |