A preliminary trial: double-blind comparison of nefazodone, bupropion-SR, and placebo in the treatment of cannabis dependence.
Kenneth M Carpenter, David McDowell, Daniel J Brooks, Wendy Y Cheng, Frances R Levin
Index: Am. J. Addict. 18(1) , 53-64, (2009)
Full Text: HTML
Abstract
The present study investigated the efficacy of nefazodone and bupropion-sustained release for treating cannabis dependence. A double-blind, placebo-controlled, piggy back design was employed to assess if nefazodone and bupropion-sustained release increased the probability of abstinence from cannabis and reduced the severity of cannabis dependence and cannabis withdrawal symptoms during a 13-week outpatient treatment program. One-hundred and six participants (Mean = 32 years; females n = 25) were randomized to one of three medication conditions (nefazodone, bupropion-sustained release, or placebo) and participated in a weekly, individually based coping skills therapy program. Results indicated an increased probability of achieving abstinence over the course of treatment and a decrease in the severity of cannabis dependence and the withdrawal symptom of irritability. There were no significant effects demonstrated for nefazodone and bupropion-sustained release on cannabis use or cannabis withdrawal symptoms. The results indicate nefazodone and bupropion-sustained release may have limited efficacy in treating cannabis dependence.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
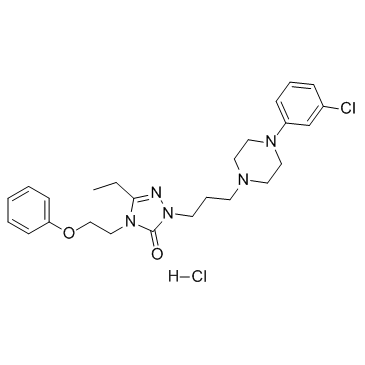 |
Nefazodone hydrochloride
CAS:82752-99-6 |
C25H33Cl2N5O2 |
|
Systematic evaluation of commercially available ultra-high p...
2014-12-29 [J. Chromatogr. A. 1374 , 122-33, (2014)] |
|
The relation between changes in patients' interpersonal impa...
2012-06-01 [J. Consult. Clin. Psychol. 80(3) , 354-64, (2012)] |
|
Comparison of the bioactivation potential of the antidepress...
2008-06-01 [Drug Metab. Dispos. 36(6) , 1016-29, (2008)] |
|
Inhibition of G protein-activated inwardly rectifying K+ cha...
2011-01-01 [PLoS ONE 6(12) , e28208, (2011)] |
|
Dyadic discord at baseline is associated with lack of remiss...
2010-03-01 [Psychol. Med. 40(3) , 415-24, (2010)] |