| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
4-Hydroxyindole
CAS:2380-94-1 |
|
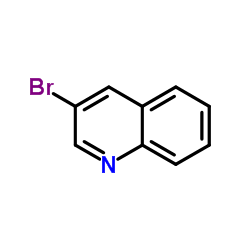 |
3-Bromoquinoline
CAS:5332-24-1 |
|
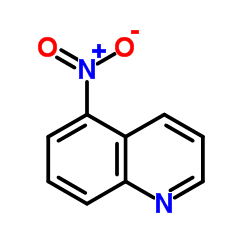 |
5-Nitroquinoline
CAS:607-34-1 |
|
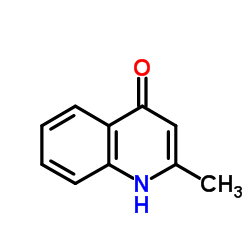 |
2-Methyl-4-quinolinol
CAS:607-67-0 |
|
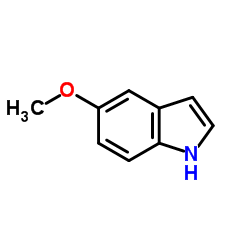 |
5-Methoxyindole
CAS:1006-94-6 |
|
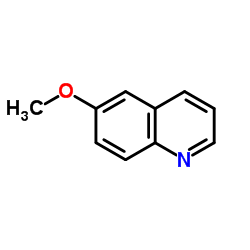 |
6-Methoxyquinoline
CAS:5263-87-6 |
|
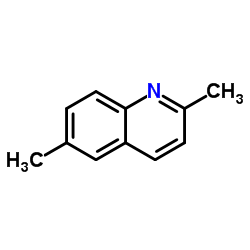 |
2,6-Dimethylquinoline
CAS:877-43-0 |
|
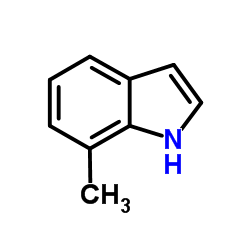 |
7-Methylindole
CAS:933-67-5 |
|
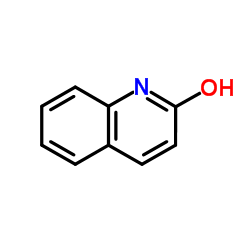 |
quinolone
CAS:59-31-4 |