Positional adaptability in the design of mutation-resistant nonnucleoside HIV-1 reverse transcriptase inhibitors: a supramolecular perspective.
Aldo Bruccoleri
Index: AIDS Res. Hum. Retroviruses 29(1) , 4-12, (2013)
Full Text: HTML
Abstract
Drug resistance is a key cause of failed treatment of HIV infection. The efficacy of nonnucleoside reverse transcriptase-inhibiting (NNRTI) drugs is impaired by the rapid emergence of drug-resistant mutations. The literature supports the idea that purposefully designed flexible NNRTIs at an active site may help overcome drug resistance. It is proposed here that the usual "lock and key" model, with respect to NNRTI drug design, be expanded to consider creating "master keys" that would automatically adjust conformations to fit all of the "locks" mutations may make. The present work introduces the novel perspective of designing and creating supramolecular assemblies as potential NNRTIs (instead of the relatively more rigid single-molecule inhibitors). Specifically, flexible self-assembling quinhydrone supramolecular dimers formed from quinonoid monomers (designed to be highly flexible NNRTIs themselves) will be offered as a working example of this new perspective in NNRTI drug design. Quinonoid compounds have demonstrated binding interactions at various sites of the HIV-1 RT enzyme, including the elusive ribonuclease H area. Quinhydrone self-organized dimers have at some point in their molecular architecture a noncovalently interacting donor-acceptor ring pair complex. This complex is at the heart of the increased torsional, rotational, and translational motion this species will experience at a particular active site. Flexible supramolecular assemblies, together with their flexible monomer components, may offer a critical advantage in retaining potency against a wide range of drug-resistant HIV-1 RTs. This new supramolecular perspective may also have broader implications in the general field of antimicrobial drug design.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
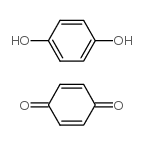 |
Quinhydrone
CAS:106-34-3 |
C12H10O4 |
|
Tenebrionid secretions and a fungal benzoquinone oxidoreduct...
2015-07-14 [Proc. Natl. Acad. Sci. U. S. A. 112 , E3651-60, (2015)] |
|
The effect of the hydrogen ion concentration upon the salt e...
1947-03-01 [J. Am. Chem. Soc. 69(3) , 533-6, (1947)] |
|
[Not Available].
1947-06-01 [Z. Gesamte Inn. Med. 2(11-12) , 333-46, (1947)] |
|
A quinhydrone-type 2 ratio 1 acceptor-donor charge transfer ...
2006-04-28 [Chem. Commun. (Camb.) (16) , 1751-3, (2006)] |
|
Disulfide bond formation involves a quinhydrone-type charge-...
2003-11-25 [Proc. Natl. Acad. Sci. U. S. A. 100(24) , 13779-84, (2003)] |