Preliminary investigation of naringenin hydroxylation with recombinant E. coli expressing plant flavonoid hydroxylation gene.
Ilef Limem-Ben Amor, Nidhal Salem, Emmanuel Guedon, Jean-Marc Engasser, Leila Chekir-Ghedrira, Mohamed Ghoul
Index: Nat. Prod. Commun. 5(5) , 777-82, (2010)
Full Text: HTML
Abstract
Flavonoid hydroxylation is one way to increase the biological activities of these molecules and the number of hydroxyl groups needed for polymerization, esterification, alkylation, glycosylation and acylation reactions. These reactions have been suggested as a promising route to enhance flavonoid solubility and stability. In our preliminary study we hydroxylated naringenin (the first flavonoid core synthesized in plants) with recombinant E. coli harboring flavanone 3 hydroxylase (F3H). We demonstrated that recombinant E. coli harboring the F3H from Petroselinum crispum, can convert naringenin to dihydrokaempferol. The whole cell hydroxylase activity was often influenced by the stability of the plasmid harboring the cloned gene and the biomass yield. When the composition of the growth media became richer the amount of formed product decreased about twofold; the naringenin bioconversion yield in LB media was 70% and decreased to 33% in TB. However, the enrichment of culture media increased the biomass yield nearly threefold in LB media, only 0.5 g/L of bacteria was formed, but in TB there was 1.6 g/L. Thus, LB constitutes the best medium for naringenin bioconversion using the recombinant E. coli harboring the F3H; this allows for maximum bioconversion yield and plasmid stability when compared with the fourth tested culture medium. Consequently, E. coli harboring F3H from Petroselinum crispum can be used to produce flavonoids hydroxylated in position 3 that can serve in additional reactions like polymerization, glycosylation, and acylation,
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
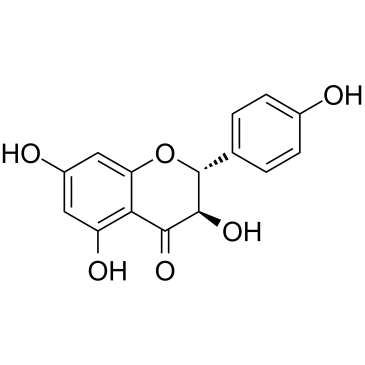 |
Aromadendrin
CAS:480-20-6 |
C15H12O6 |
|
Transcriptome sequencing and metabolite analysis reveals the...
2014-07-01 [J. Exp. Bot. 65(12) , 3157-64, (2014)] |
|
Functional characterization of a Plagiochasma appendiculatum...
2014-06-27 [FEBS Lett. 588(14) , 2307-14, (2014)] |
|
Production of 7-O-methyl aromadendrin, a medicinally valuabl...
2012-02-01 [Appl. Environ. Microbiol. 78(3) , 684-94, (2012)] |
|
5-O-glucosyldihydroflavones from the leaves of Helicia cochi...
2006-12-01 [Phytochemistry 67(24) , 2681-5, (2006)] |
|
7-O-methylaromadendrin stimulates glucose uptake and improve...
2010-01-01 [Biol. Pharm. Bull. 33(9) , 1494-9, (2010)] |