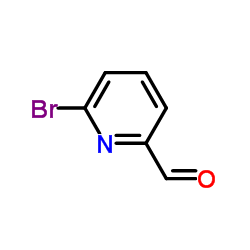
|
~82% |
|
~% |
|
~% |
|
~94% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~98% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
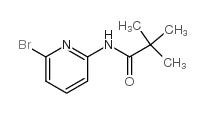
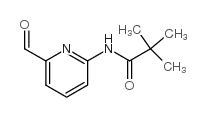
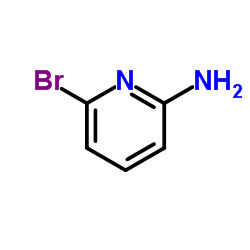
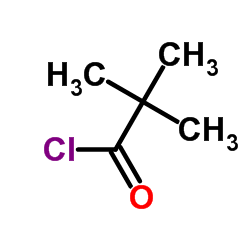

![4-(TERT-BUTOXYCARBONYLAMINO)-1-[(6-BROMOPYRIDIN-2-YL)METHYL]PIPERIDINE Structure](https://image.chemsrc.com/caspic/081/303763-37-3.png)
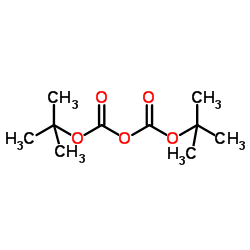


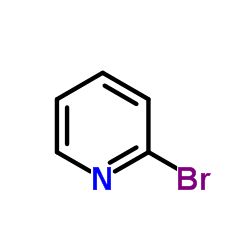
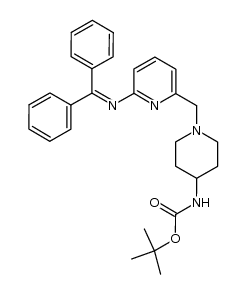
![4-(TERT-BUTOXYCARBONYLAMINO)-1-[(6-AMINOPYRIDIN-2-YL)METHYL]PIPERIDINE Structure](https://image.chemsrc.com/caspic/005/303763-39-5.png)