| Structure | Name/CAS No. | Articles |
|---|---|---|
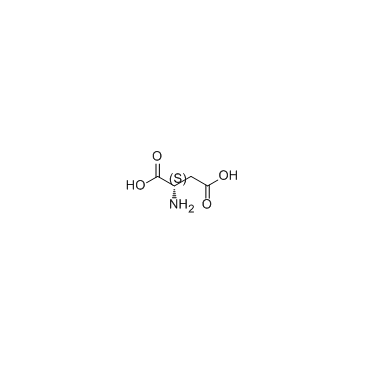 |
L-Aspartic acid
CAS:56-84-8 |
|
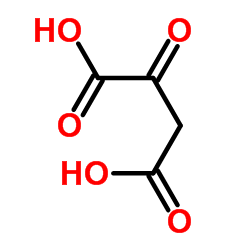 |
Oxaloacetic acid
CAS:328-42-7 |
|
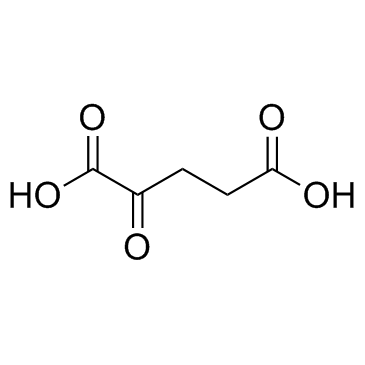 |
2-Ketoglutaric acid
CAS:328-50-7 |
|
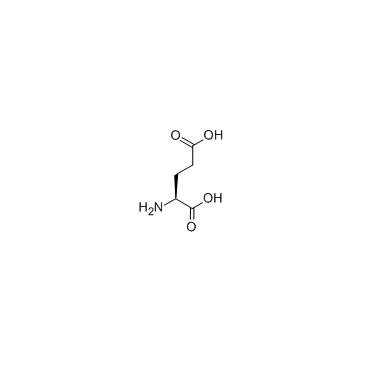 |
L-glutamic acid
CAS:56-86-0 |
|
 |
L-Phenylalanine
CAS:63-91-2 |
|
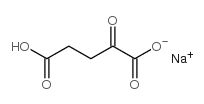 |
Alpha-Ketoglutaric acid sodium salt
CAS:22202-68-2 |
|
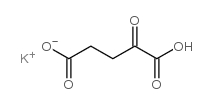 |
Potassium hydrogen 2-oxoglutarate
CAS:997-43-3 |
|
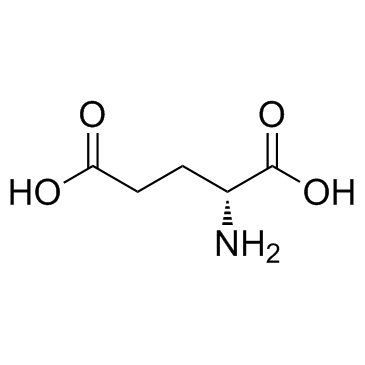 |
D(-)-Glutamic acid
CAS:6893-26-1 |
|
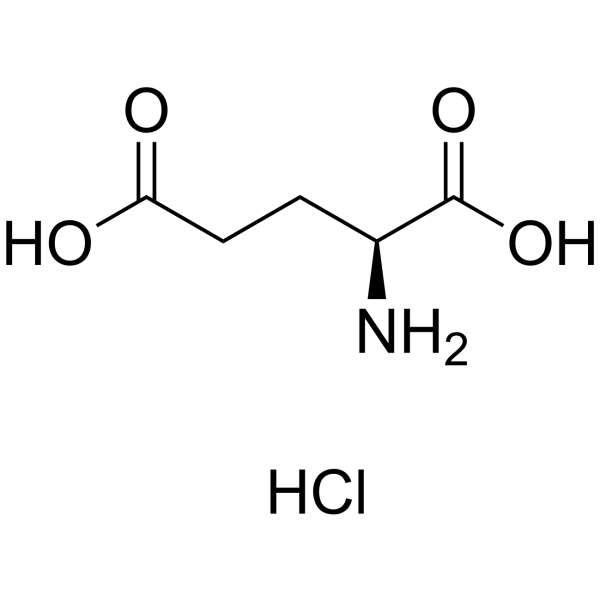 |
L-Glutamic acid:Hcl (17O4)
CAS:138-15-8 |