Organic Letters
2006-10-12
Stereochemical control in the reduction of 2-chromanols.
Kelin Li, Kumar Vanka, Ward H Thompson, Jon A Tunge
Index: Org. Lett. 8 , 4711, (2006)
Full Text: HTML
Abstract
[reaction: see text] Reduction of C5-substituted 2-hydroxychromans selectively provides 2,4-cis-chromans using large silane reductants and 2,4-trans-chromans using the smaller silane PhSiH(3). The stereochemical outcome has been rationalized on the basis of a Curtin-Hammett kinetic situation arising from hydride delivery to two different conformations of an intermediate oxocarbenium ion. This method provides a powerful way to control the relative stereochemistry of these substructures which are prevalent in bioactive natural products.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
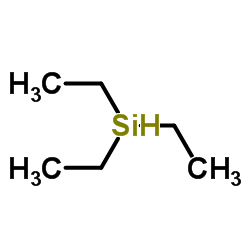 |
Triethylsilane
CAS:617-86-7 |
C6H16Si |
Related Articles:
More...
|
Colloidal nanocrystals of lithiated group 14 elements.
2014-12-22 [Angew. Chem. Int. Ed. Engl. 53(52) , 14527-32, (2014)] |
|
Discovery of XEN907, a spirooxindole blocker of NaV1.7 for t...
2011-01-01 [Bioorg. Med. Chem. Lett. 21 , 3676-3681, (2011)] |
|
Discovery of piperazin-1-ylpyridazine-based potent and selec...
2013-01-24 [J. Med. Chem. 56(2) , 568-83, (2013)] |
|
Multi-modal detection of colon malignancy by NIR-tagged reco...
2015-01-30 [Int. J. Pharm. 478(2) , 504-16, (2015)] |
|
Nonradioactive, ultrasensitive site-specific protein-protein...
2008-11-01 [Nucleic Acids Res. 36(19) , 6143-54, (2008)] |