| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Native Porcine Angiotensin Converting Enzyme
CAS:9015-82-1 |
|
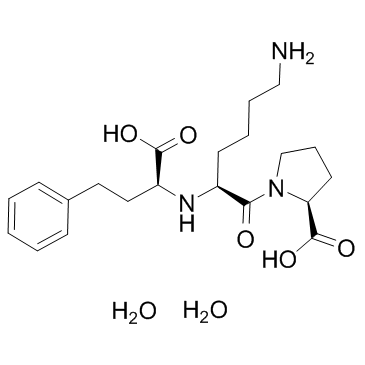 |
Lisinopril diydrate
CAS:83915-83-7 |