| Structure | Name/CAS No. | Articles |
|---|---|---|
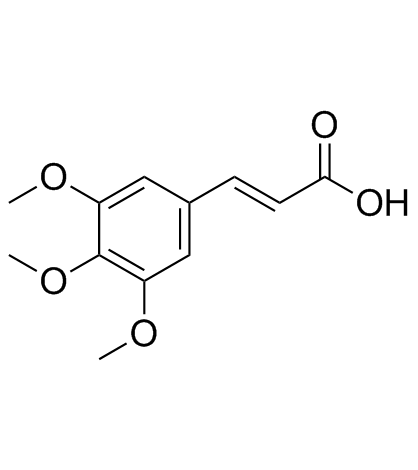 |
3,4,5-Trimethoxycinnamic acid
CAS:90-50-6 |
Yingming Wu, Jingfeng Zhao, Jingbo Chen, Chengxue Pan, Liang Li, Hongbin Zhang
Index: Org. Lett. 11(3) , 597-600, (2009)
Full Text: HTML
A highly flexible and concise total synthesis of (+)-podophyllotoxin featured with an enantioselective sequential conjugate addition-allylation reaction was reported. Starting from commercially available 3,4,5-trimethoxycinnamic acid, this new route leads to (+)-podophyllotoxin 1 in only eight steps with 29% overall yield.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
3,4,5-Trimethoxycinnamic acid
CAS:90-50-6 |
C12H14O5 |
|
A metabolite profiling approach to identify biomarkers of fl...
2009-12-01 [J. Nucl. Med. 139 , 2309-14, (2009)] |
|
Human fecal water content of phenolics: the extent of coloni...
2005-03-15 [Free Radic. Biol. Med. 38(6) , 763-72, (2005)] |
|
[The detection of Azotobacter and its significance in crimin...
1980-01-01 [Arch. Kriminol. 165(1-2) , 27-34, (1980)] |
|
Biosynthesis of podophyllotoxin in Linum album cell cultures...
2002-10-01 [Planta 215(6) , 1031-9, (2002)] |
|
High-performance liquid chromatographic method for screening...
[J. Chromatogr. A. 310(2) , 273-81, (1984)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved