| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Palladium
CAS:7440-05-3 |
|
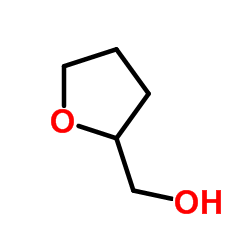 |
Tetrahydrofurfuryl alcohol
CAS:97-99-4 |
|
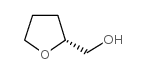 |
(R)-(-)-TETRAHYDROFURFURYLALCOHOL
CAS:22415-59-4 |
|
 |
Cyclopentenone
CAS:930-30-3 |