Enhanced transdermal delivery of evodiamine and rutaecarpine using microemulsion.
Yong-Tai Zhang, Ji-Hui Zhao, Su-Juan Zhang, Yang-Zi Zhong, Zhi Wang, Ying Liu, Feng Shi, Nian-Ping Feng
Index: Int. J. Nanomedicine 6 , 2469-82, (2011)
Full Text: HTML
Abstract
The purpose of this study was to improve skin permeation of evodiamine and rutaecarpine for transdermal delivery with microemulsion as vehicle and investigate real-time cutaneous absorption of the drugs via in vivo microdialysis.Pseudoternary phase diagrams were constructed to evaluate microemulsion regions with various surfactants and cosurfactants. Nine formulations of oil in water microemulsions were selected as vehicles for assessing skin permeation of evodiamine and rutaecarpine in ex vivo transdermal experiments. With a microdialysis hollow fiber membrane implanted in the skin beneath the site of topical drug administration, dialysis sampling was maintained for 10 hours and the samples were detected directly by high performance liquid chromatography. Real-time concentrations of the drugs in rat skin were investigated and compared with those of conventional formulations, such as ointment and tincture. Furthermore, the drugs were applied to various regions of the skin using microemulsion as vehicle.In ex vivo transdermal experiments, cutaneous fluxes of evodiamine and rutaecarpine microemulsions were 2.55-fold to 11.36-fold and 1.17-fold to 6.33-fold higher, respectively, than those of aqueous suspensions. Different drug loadings, microemulsion water content, and transdermal enhancers markedly influenced the permeation of evodiamine and rutaecarpine. In microemulsion application with in vivo microdialysis, the maximum concentration of the drugs (evodiamine: 18.23 ± 1.54 ng/mL; rutaecarpine: 16.04 ± 0.69 ng/mL) were the highest, and the area under the curve(0-t) of evodiamine and rutaecarpine was 1.52-fold and 2.27-fold higher than ointment and 3.06-fold and 4.23-fold higher than tincture, respectively. A greater amount of drugs penetrated through and was absorbed by rat abdominal skin than shoulder and chest, and a reservoir in the skin was found to supply drugs even after the microemulsion was withdrawn.Compared to conventional formulations, higher cutaneous fluxes of evodiamine and rutaecarpine were achieved with microemulsion. Based on this novel transdermal delivery, the transdermal route was effective for the administration of the two active alkaloids.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
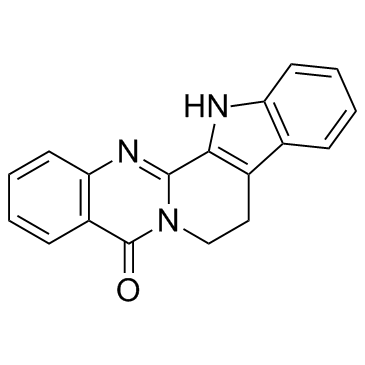 |
Rutaecarpine
CAS:84-26-4 |
C18H13N3O | |
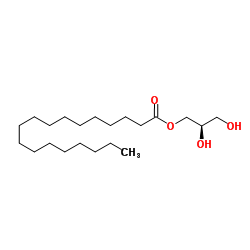 |
Monostearin
CAS:123-94-4 |
C21H42O4 |
|
Pharmacological effects of rutaecarpine as a cardiovascular ...
2010-03-01 [Molecules 15(3) , 1873-81, (2010)] |
|
Transdermal behaviors comparisons among Evodia rutaecarpa ex...
2012-07-01 [Fitoterapia 83(5) , 954-60, (2012)] |
|
Effects of rutaecarpine on the metabolism and urinary excret...
2011-01-01 [Arch. Pharm. Res. 34(1) , 119-25, (2011)] |
|
Synthesis and biological properties of benzo-annulated rutae...
2010-01-01 [Biol. Pharm. Bull. 33(10) , 1704-9, (2010)] |
|
Rutaecarpine prevents hypoxia-reoxygenation-induced myocardi...
2011-03-01 [Can. J. Physiol. Pharmacol. 89(3) , 177-86, (2011)] |