Biological & Pharmaceutical Bulletin
2010-01-01
Synthesis and biological properties of benzo-annulated rutaecarpines.
Young Hwan Hong, Woo Jin Lee, Seung Ho Lee, Jong Keun Son, Hye-Lin Kim, Jung Min Nam, Youngjoo Kwon, Yurngdong Jahng
Index: Biol. Pharm. Bull. 33(10) , 1704-9, (2010)
Full Text: HTML
Abstract
A series of benzo-annulated rutaecarpines were prepared from anthranilic acid and 3-aminonaphthalene-2-carboxylic acid by Fischer indole synthesis as key reaction. Cytotoxicity was somewhat increased by the introduction of benzo-annulation, which was not directly related to the inhibitory activity against topoisomerases (topo) I and II. Benzo-annulation on ring A led to significant increase of inhibitory activity against topo II while annulations on ring E increased inhibitory activity against topo I.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
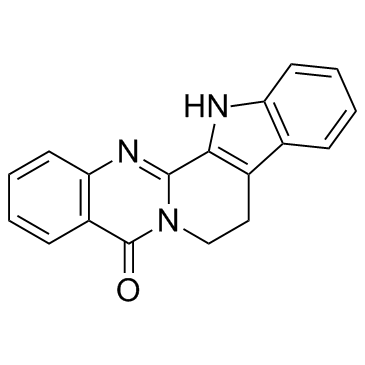 |
Rutaecarpine
CAS:84-26-4 |
C18H13N3O |
Related Articles:
More...
|
Pharmacological effects of rutaecarpine as a cardiovascular ...
2010-03-01 [Molecules 15(3) , 1873-81, (2010)] |
|
Transdermal behaviors comparisons among Evodia rutaecarpa ex...
2012-07-01 [Fitoterapia 83(5) , 954-60, (2012)] |
|
Effects of rutaecarpine on the metabolism and urinary excret...
2011-01-01 [Arch. Pharm. Res. 34(1) , 119-25, (2011)] |
|
Rutaecarpine prevents hypoxia-reoxygenation-induced myocardi...
2011-03-01 [Can. J. Physiol. Pharmacol. 89(3) , 177-86, (2011)] |
|
A new quinolone and other constituents from the fruits of Te...
2010-07-01 [Chem. Biodivers. 7(7) , 1828-34, (2010)] |