| Structure | Name/CAS No. | Articles |
|---|---|---|
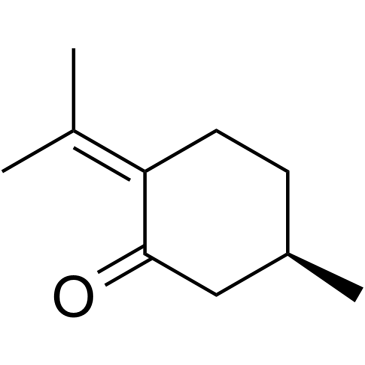 |
Pulegone
CAS:89-82-7 |
|
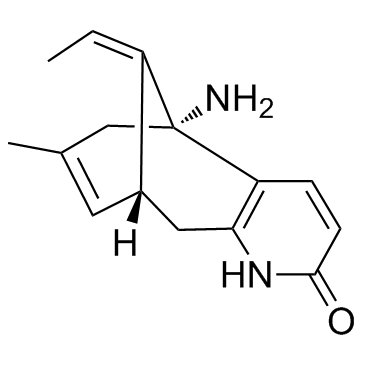 |
(-)-Huperzine A
CAS:102518-79-6 |
|
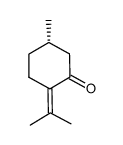 |
(S)-(-)-Pulegone
CAS:3391-90-0 |