Dichloro-bis(aminophosphine) complexes of palladium: highly convenient, reliable and extremely active suzuki-miyaura catalysts with excellent functional group tolerance.
Jeanne L Bolliger, Christian M Frech
Index: Chemistry 16(13) , 4075-81, (2010)
Full Text: HTML
Abstract
Dichloro-bis(aminophosphine) complexes are stable depot forms of palladium nanoparticles and have proved to be excellent Suzuki-Miyaura catalysts. Simple modifications of the ligand (and/or the addition of water to the reaction mixture) have allowed their formation to be controlled. Dichlorobis[1-(dicyclohexylphosphanyl)piperidine]palladium (3), the most active catalyst of the investigated systems, is a highly convenient, reliable, and extremely active Suzuki catalyst with excellent functional group tolerance that enables the quantitative coupling of a wide variety of activated, nonactivated, and deactivated and/or sterically hindered functionalized and heterocyclic aryl and benzyl bromides with only a slight excess (1.1-1.2 equiv) of arylboronic acid at 80 degrees C in the presence of 0.2 mol % of the catalyst in technical grade toluene in flasks open to the air. Conversions of >95 % were generally achieved within only a few minutes. The reaction protocol presented herein is universally applicable. Side-products have only rarely been detected. The catalytic activities of the aminophosphine-based systems were found to be dramatically improved compared with their phosphine analogue as a result of significantly faster palladium nanoparticle formation. The decomposition products of the catalysts are dicyclohexylphosphinate, cyclohexylphosphonate, and phosphate, which can easily be separated from the coupling products, a great advantage when compared with non-water-soluble phosphine-based systems.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
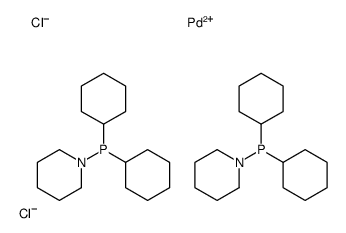 |
Dichlorobis(dicyclohexyl-1-piperidinylphosphine)palladium(II)
CAS:1227935-55-8 |
C34H64Cl2N2P2Pd |
|
Cyanation of aryl bromides with K4[Fe(CN)6] catalyzed by dic...
2012-03-05 [Chemistry 18(10) , 2978-86, (2012)] |
|
Alkyne hydrothiolation catalyzed by a dichlorobis(aminophosp...
2012-07-16 [Chemistry 18(29) , 8901-5, (2012)] |
|
[Pd(Cl)2{P(NC5H10)(C6H11)2}2]--a highly effective and extrem...
2010-09-24 [Chemistry 16(36) , 11072-81, (2010)] |
|
Comparative Study of the Frech Catalyst with Two Conventiona...
2013-06-01 [Org. Prep. Proced. Int. 45(1) , 66-71, (2013)] |
|
Access to 2-Aminopyridines - Compounds of Great Biological a...
[Adv. Synth. Catal. 353 , 945, (2011)] |