| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Erythromycin
CAS:114-07-8 |
|
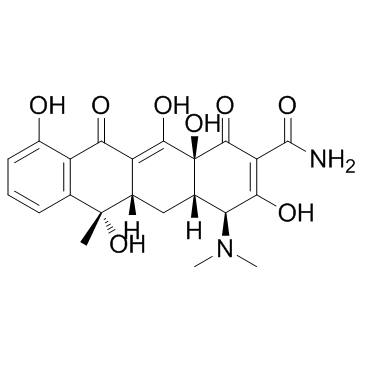 |
Tetracycline
CAS:60-54-8 |
|
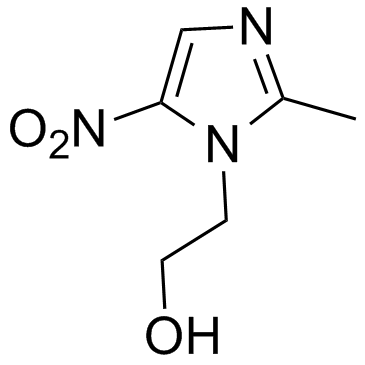 |
Metronidazole
CAS:443-48-1 |
|
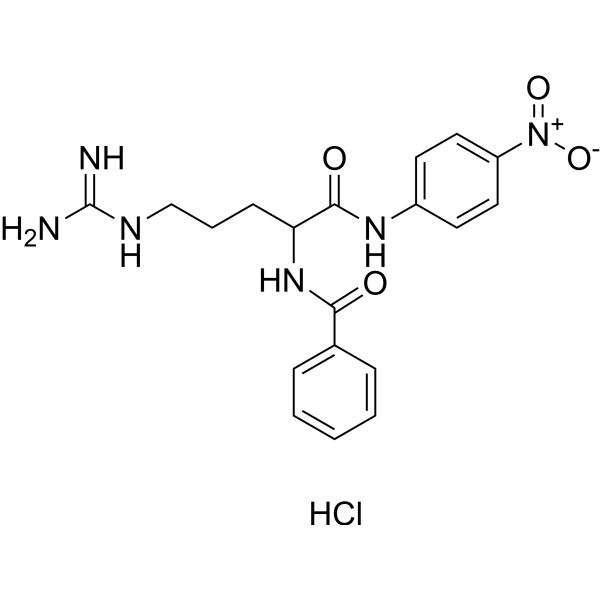 |
N-Benzoyl-DL-arginine-4-nitroanilide hydrochloride
CAS:911-77-3 |
|
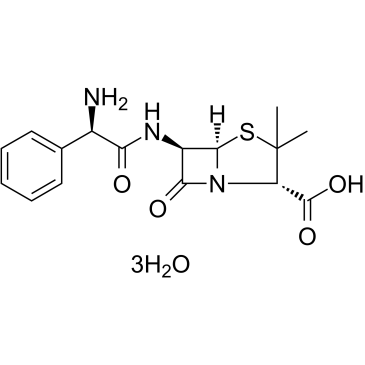 |
Ampicillin Trihydrate
CAS:7177-48-2 |
|
 |
Ampicillin
CAS:69-53-4 |
|
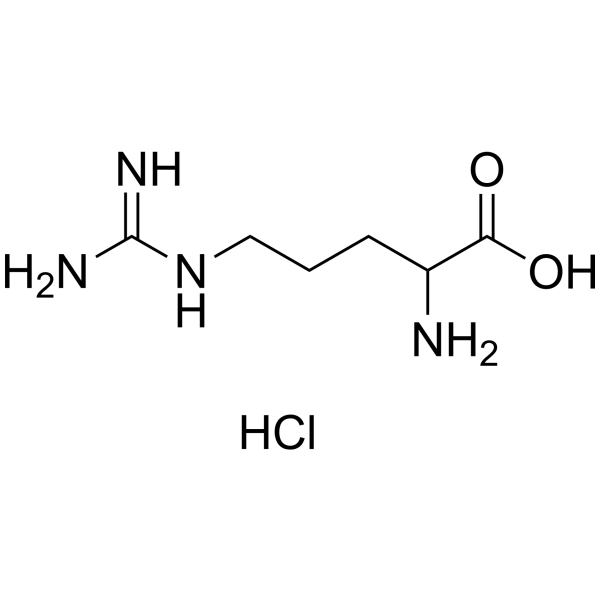 |
H-DL-Arg-OH.HCl
CAS:32042-43-6 |