| Structure | Name/CAS No. | Articles |
|---|---|---|
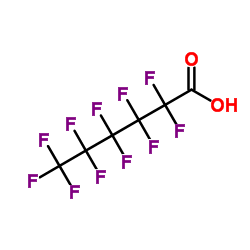 |
Perfluorohexanoic acid
CAS:307-24-4 |
|
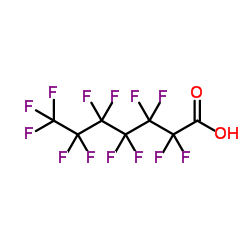 |
Perfluoroheptanoic acid
CAS:375-85-9 |
|
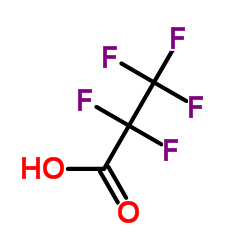 |
Pentafluoropropanoic acid
CAS:422-64-0 |