Cross-species comparisons of the pharmacokinetics of ibudilast.
L M Sanftner, J A Gibbons, M I Gross, B M Suzuki, F C A Gaeta, K W Johnson
Index: Xenobiotica 39(12) , 964-77, (2009)
Full Text: HTML
Abstract
To enable clinical development of ibudilast for new indications, its pharmacokinetics were characterized in mice, rats, rabbits, dogs, cynomolgus monkeys, and minipigs. Animal pharmacokinetics were compared with a separate study in healthy volunteers. Following oral dosing, the dose-normalized area under the curve (AUC) (DN-AUC(24h)) in humans is 896 ((ng*h ml(-1))/(mg kg(-1))), and in animals ranges from 0.3 to 87. The variability among species cannot be explained by intrinsic clearance, which in intravenous dosing experiments shows only moderate interspecies variation (13-41 l h(-1) m(-2)). A portal vein rat pharmacokinetics model suggested that differences in first-pass gut clearance may explain some of the interspecies variation in oral bioavailability. Ibudilast shows auto-induction of metabolism in some animals, but not in humans. Plasma protein binding in humans and some animals is greater than or equal to 95%. The primary metabolite 6,7-dihyrdodiol-ibudilast is measurable and has been quantitated in plasma from animals and humans. Finally, biodistribution studies show that ibudilast distributes rapidly, extensively, and reversibly to the central nervous system.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
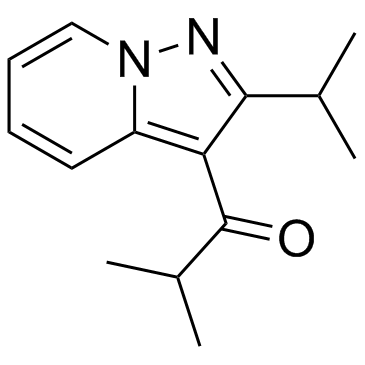 |
Ibudilast
CAS:50847-11-5 |
C14H18N2O |
|
The glial cell modulators, ibudilast and its amino analog, A...
2012-03-15 [Eur. J. Pharmacol. 679(1-3) , 75-80, (2012)] |
|
Phosphodiesterase inhibitors. Part 1: Synthesis and structur...
2011-01-01 [Bioorg. Med. Chem. Lett. 21(11) , 3307-12, (2011)] |
|
Ibudilast, a pharmacologic phosphodiesterase inhibitor, prev...
2011-01-01 [PLoS ONE 6(4) , e18633, (2011)] |
|
Inhibition of paclitaxel-induced decreases in calcium signal...
2012-11-02 [J. Biol. Chem. 287(45) , 37907-16, (2012)] |
|
Ibudilast, a mixed PDE3/4 inhibitor, causes a selective and ...
2011-01-15 [Eur. J. Pharmacol. 650(2-3) , 605-11, (2011)] |