| Structure | Name/CAS No. | Articles |
|---|---|---|
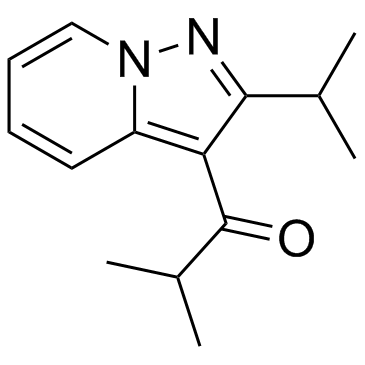 |
Ibudilast
CAS:50847-11-5 |
|
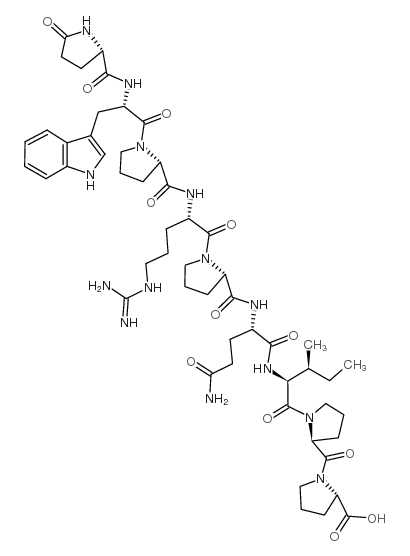 |
BPP 9a
CAS:35115-60-7 |