| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium dodecyl sulfate
CAS:151-21-3 |
|
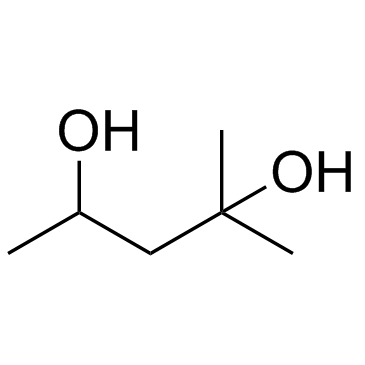 |
Hexylene glycol
CAS:107-41-5 |
|
 |
Lysozyme
CAS:12650-88-3 |
|
 |
Lysozyme
CAS:9001-63-2 |
|
 |
Lysozyme hydrochloride
CAS:9066-59-5 |
|
 |
Human milk lysozyme
CAS:12671-19-1 |